-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Register now
AI & ML in action: Innovations in clinical trial enrollmentThursday, April 10, 202512 PM PT / 2 PM CT / 3 PM ET
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
10/26/2022
How to use RWD for good with Najat Khan, Ph.D
Check out how Janssen’s R&D Data Science team is using RWD and AI to bring more targeted treatments to patients, saving lives.
Authors
Ryan Fukushima
COO, Tempus

Najat Khan, Ph.D.
Chief Data Science Officer and Global Head, Strategy and Operations, Janssen Research & Development

COO, Tempus

Najat Khan, Ph.D.
Chief Data Science Officer and Global Head, Strategy and Operations, Janssen Research & Development

Ryan Fukushima, COO of Tempus, met with Najat Khan, Ph.D., Chief Data Science Officer and Global Head, Strategy and Operations, R&D, The Janssen Pharmaceutical Companies of Johnson & Johnson, to talk about how Janssen’s R&D Data Science team is using real-world data (RWD) and artificial intelligence (AI) to better understand diseases and the patients impacted by them, with the goal of developing more targeted treatments. Read below to learn more about how Janssen is leveraging the power of data science and digital health to improve patient outcomes.
Questions and responses have been edited for clarity and length.
Ryan: How have real-world data-enabled algorithms helped R&D thus far? |
| Dr. Khan: We already have targeted therapies for bladder cancer patients with mutations such as FGFR alterations. However, the challenge is that most patients don’t have their tumors sequenced today, delaying access to targeted treatments.
To address this problem, Janssen looked at routinely collected histopathology data and built an algorithm that aims to predict the presence of tumor mutations from the digitized histopathology images – and we’re now deploying these algorithms to guide patient screening efforts for our clinical trials. If you can screen patients for a trial efficiently, you’ll enroll more of the right patients in the right studies. |
Ryan: How else have real-world data-enabled algorithms helped R&D thus far? |
| Dr. Khan: Patients with pulmonary hypertension are often misdiagnosed many times over extended periods of time. Think about what that does to a patient: the uncertainty when you can’t breathe properly and you aren’t receiving the care you need.
We worked with partners to develop an algorithm, which recently received FDA Breakthrough Device designation, that aims to detect subtleties in ECGs – tests frequently done within six months of the onset of symptoms, like shortness of breath – that could suggest a patient has pulmonary hypertension. Earlier detection could avoid a several-year delay in diagnosis and potentially result in earlier access to treatments. We’re still going through the validation phases of this work. Rigor is important. But this is the kind of difference and change that we’re looking to make for science and for patients. |
Ryan: How is real-world data accelerating patient recruitment? |
| Dr. Khan: One of the challenges with precision medicine is enrolling the right patients into the right trials efficiently. Today, it typically takes months to open a trial site. However, what if researchers could look at real-world data to anticipate when – and where – patients might be eligible for certain trials? That’s a patient-centric model – using real-world data to rapidly open sites where patients are through a ‘just in time’ approach. This is also helping us increase trial diversity, so that the treatments we develop can benefit more patients. |
Learn more

Learn more
Applications for multimodal real-world data
De-risking clinical trials and unlocking new knowledge about diseases
DOWNLOAD GUIDEStay informed
Be notified whenever Tempus publishes new and relevant research, webinars, and other resources.
Sign up-
03/25/2025
AI & ML in action: Unlocking RWD with GenAI through Tempus Lens
Discover how Tempus is equipping researchers with innovative AI solutions to fully leverage the potential of multimodal data. Gain insights from a panel of leaders across healthcare and life sciences as they discuss the impact of these advanced tools on delivering insights with speed.
Watch replay
Secure your recording now. -
03/11/2025
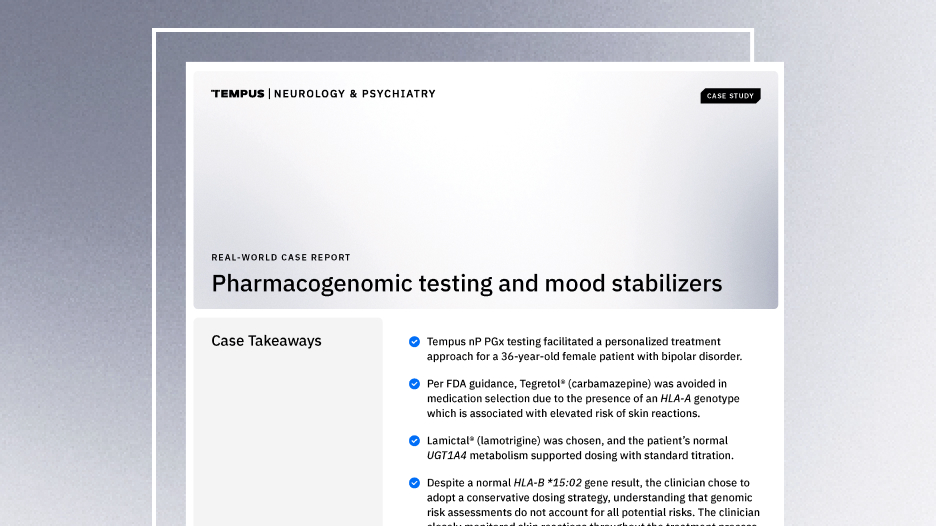
Case Report: Pharmacogenomic testing and mood stabilizers
This real-world case demonstrates how the Tempus nP pharmacogenomic test facilitated a personalized treatment approach for a patient with bipolar disorder.
Read more -
03/05/2025
Evolving outcomes research: The convergence of data, technology, and collaboration
This white paper offers an in-depth examination of the evolving field of outcomes research, focusing on the integration of real-world data and advanced analytics to improve patient outcomes. In it, we discuss the current state of the industry, the challenges of data standardization and quality, and the collaborative efforts shaping the future. Download to explore innovative approaches that are transforming outcomes research and driving the development of patient-centric healthcare solutions.
Read more