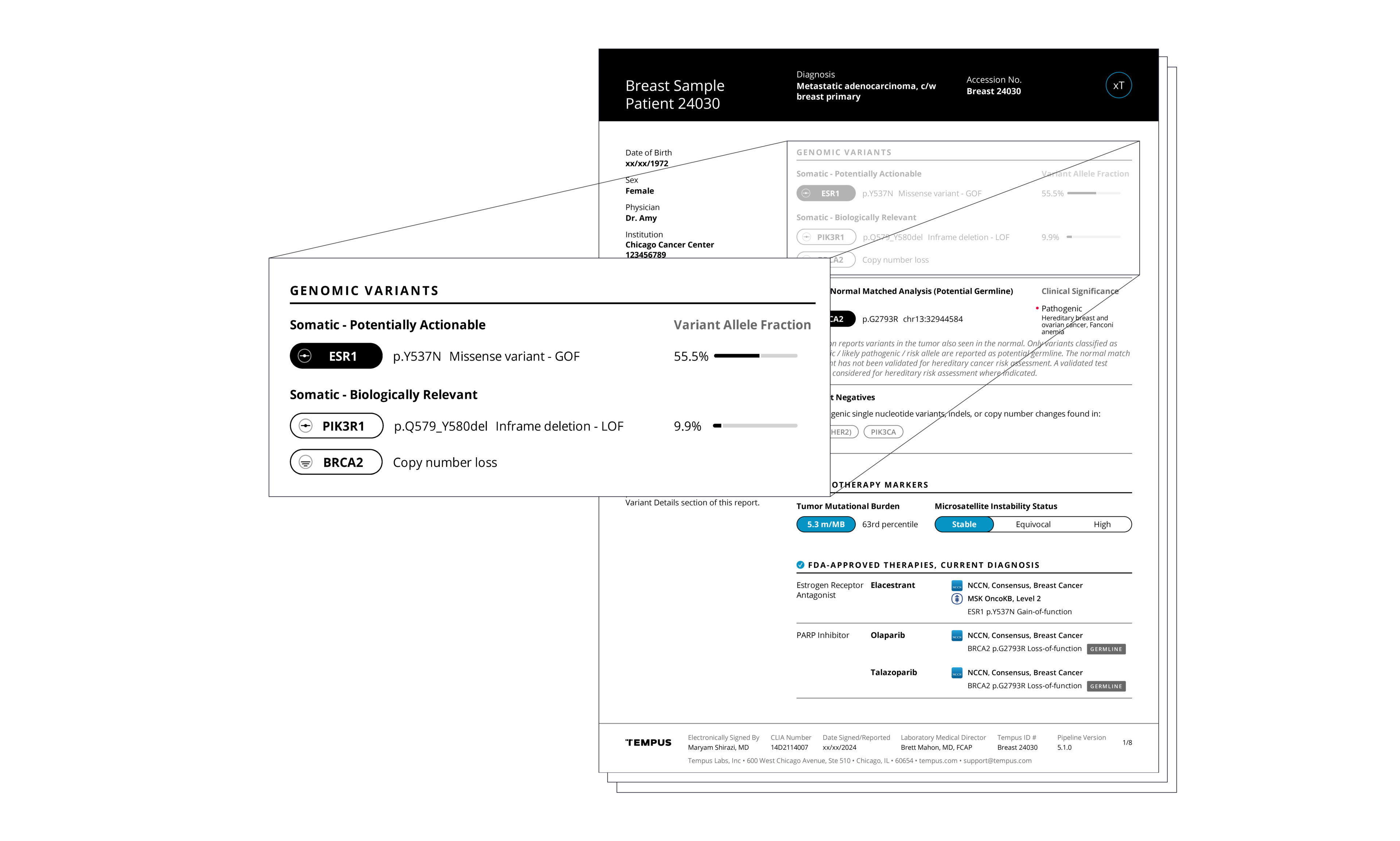
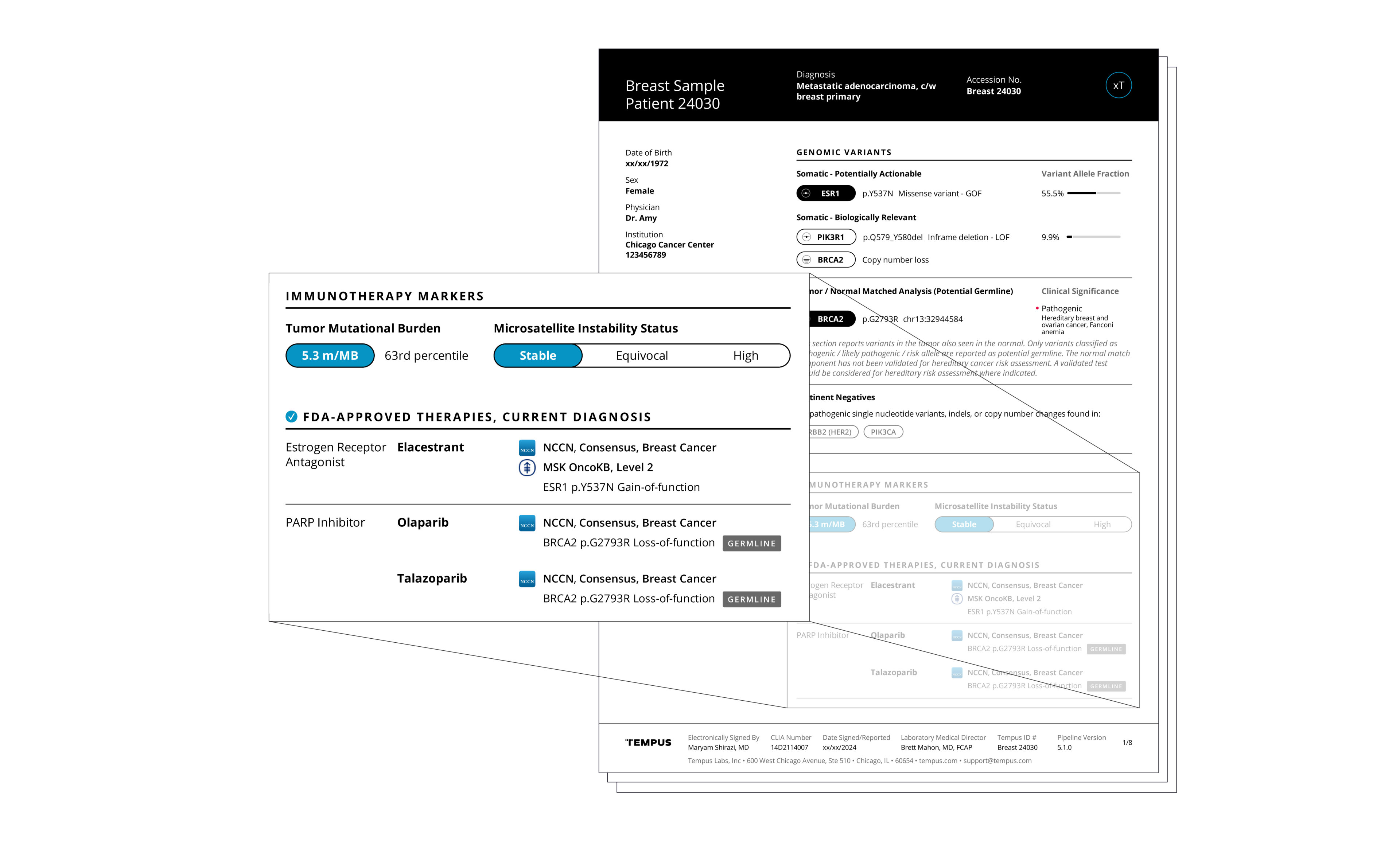
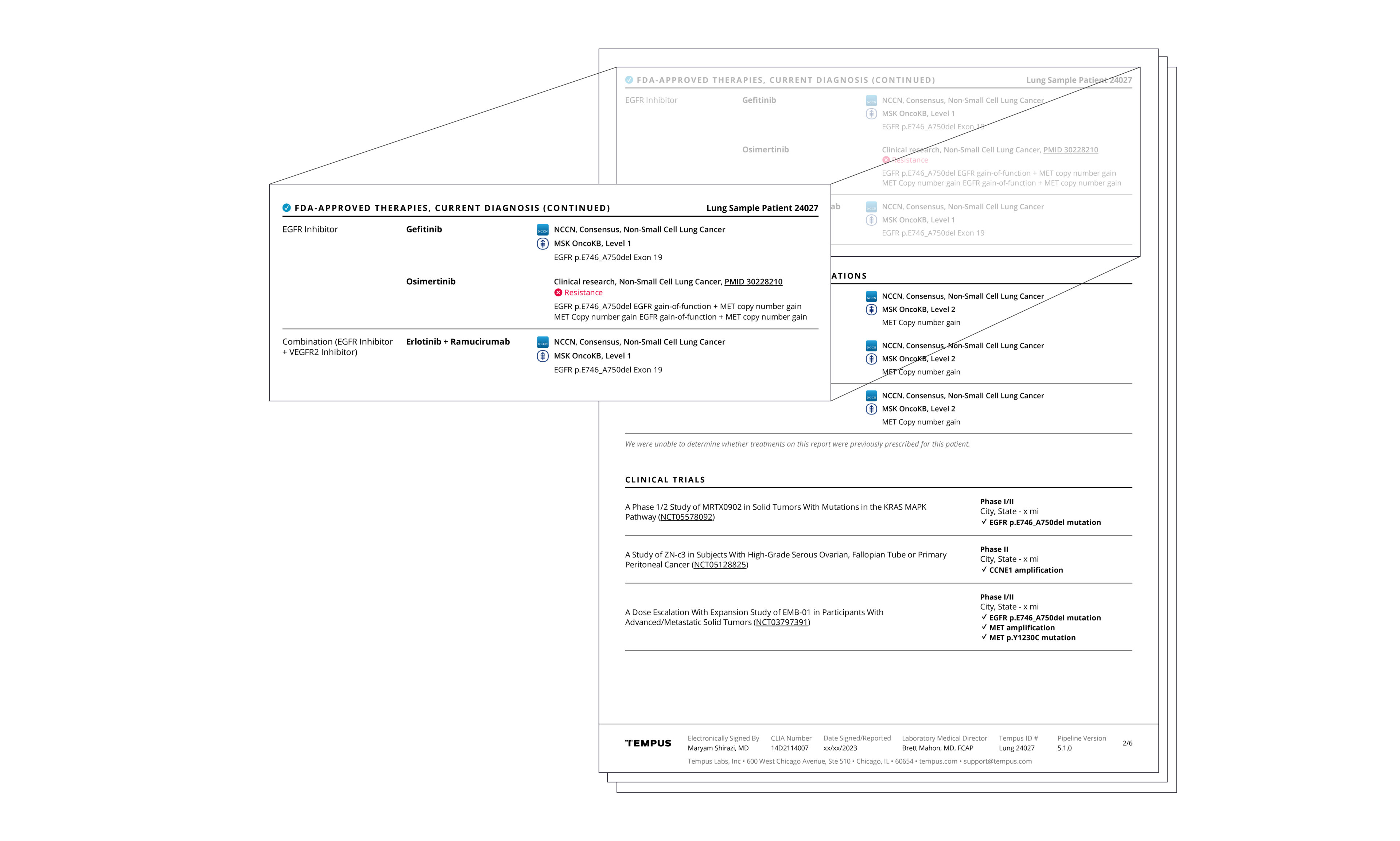
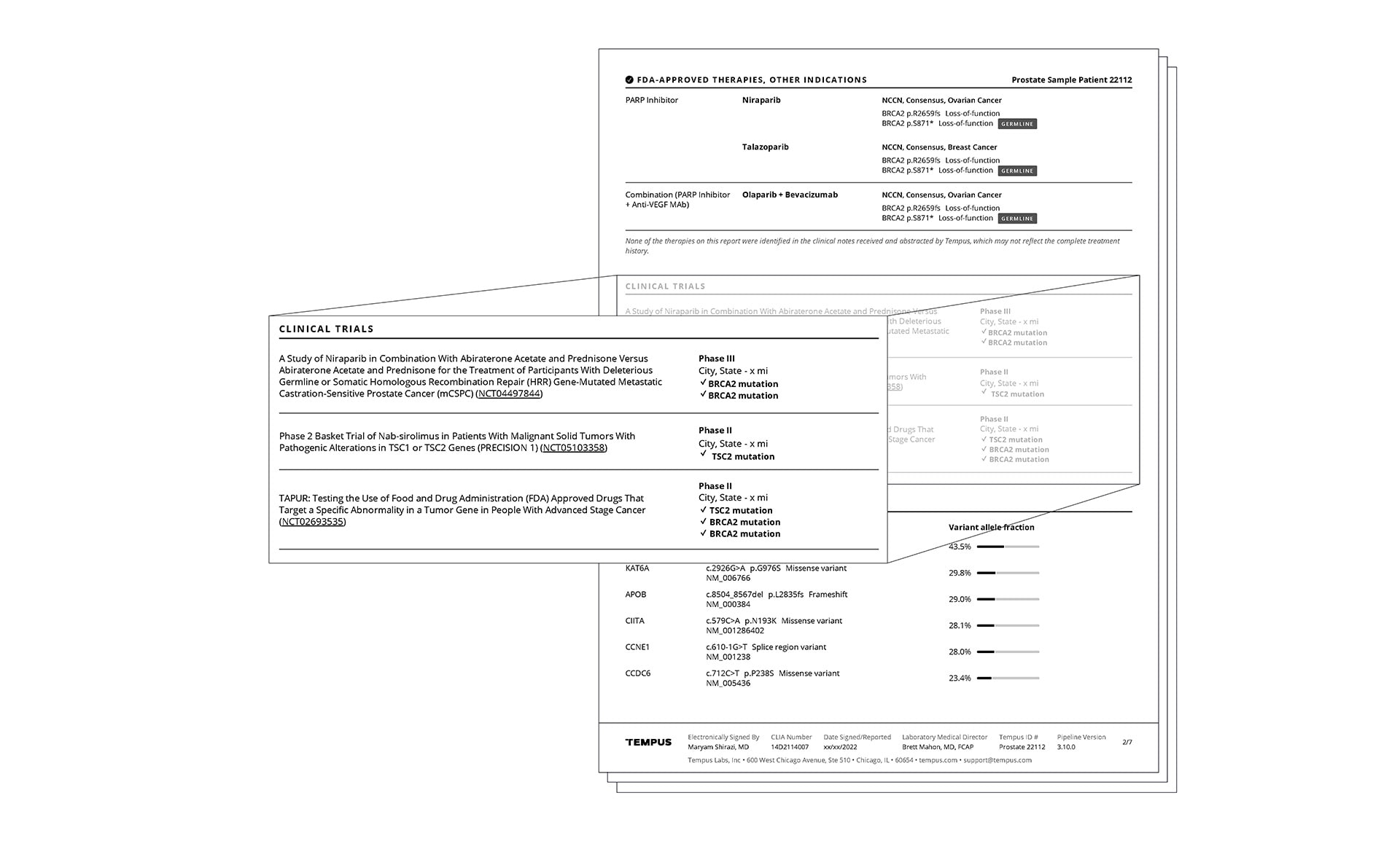
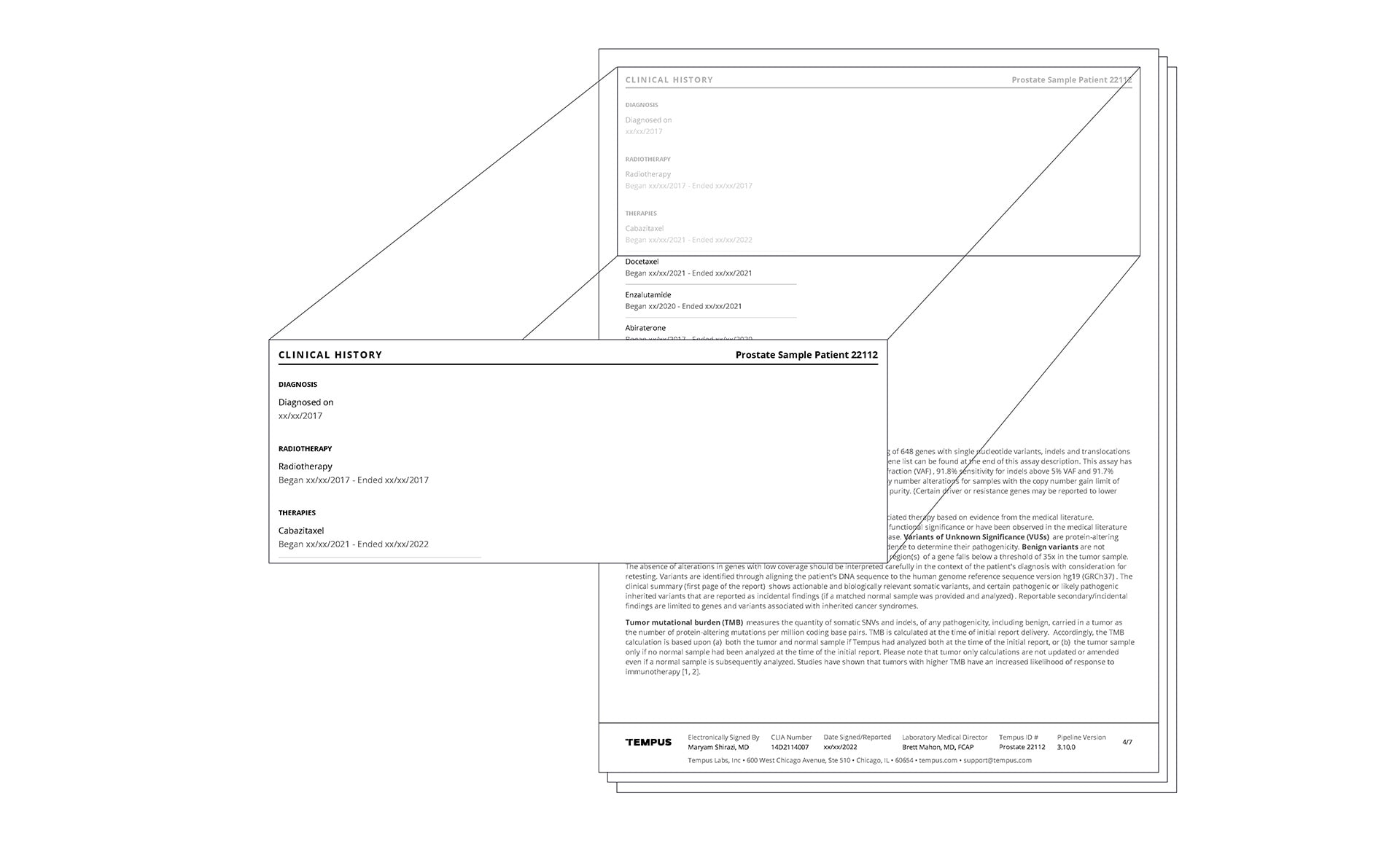
Actionable molecular findings.
-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Register now
AI & ML in action: Innovations in clinical trial enrollmentThursday, April 10, 202512 PM PT / 2 PM CT / 3 PM ET
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS