-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
07/08/2024
Tempus receives U.S. FDA 510(k) clearance for Tempus ECG-AF, an AI-based algorithm that identifies patients at increased risk of AFib
Tempus AI, Inc. (NASDAQ: TEM), a leader in AI and precision medicine, announced it has received 510(k) clearance from the FDA for its Tempus ECG-AF device. This AI-based algorithm helps identify patients who may be at increased risk of atrial fibrillation/flutter (AF), marking the first FDA clearance for an AF indication in the "cardiovascular machine learning-based notification software" category.
AF, a common cause of stroke, affects millions of people and can be challenging to diagnose. This clearance solidifies Tempus’ innovative approach to offering clinicians AI-based clinical solutions that support the potential for earlier identification of cardiovascular disease and conditions. ECG-AF is the first of a suite of next generation diagnostics that Tempus has designed to identify patients at risk for a variety of cardiovascular conditions.
Read the full press release here.
See our Research in ECG based Cardiology Algorithms:
-
-
rECHOmmend: An ECG-based machine learning approach for identifying patients at increased risk of undiagnosed structural heart disease detectable by Echocardiography: In this study, we developed a novel ECG-based machine learning approach to predict multiple structural heart conditions, hypothesizing that a composite model would yield higher prevalence and positive predictive values to facilitate meaningful recommendations for echocardiography.
-
Deep neural networks can predict new-onset Atrial Fibrillation from the 12-lead ECG and help identify those at risk of Atrial Fibrillation-related stroke (link): Atrial fibrillation (AF) is associated with substantial morbidity, especially when it goes undetected. If new-onset AF can be predicted, targeted screening could possibly be used to find it early. In this study, we explore if a deep neural network can predict new-onset AF from the resting 12-lead ECG and study whether this prediction may help identify those at risk of AF-related stroke.
-
Prospective evidence generation via ECG-AID Study: We have launched a multi-site prospective study with our beta partners to test our algorithms for atrial fibrillation and structural heart disease.
- Prediction of mortality from 12-lead electrocardiogram voltage data using a deep neural network: We hypothesized that a deep neural network (DNN) can predict an important future clinical event, 1-year all-cause mortality, from ECG voltage-time traces. In this study we used ECGs collected over a 34-year period in a large regional health system, to train a deep neural network with 1,169,662 12-lead resting ECGs obtained from 253,397 patients, in which 99,371 events occurred.
-
Our Experts |
|
Brandon Fornwalt, MD, PhDSenior Vice President of Cardiology, Tempus |
John Pfeifer, MD, MPHVice President of Clinical Cardiology, Tempus |
 |
 |
-
04/02/2025
Development of a clinical algorithm to prognosticate response to immunotherapy
Discover how Tempus developed and deployed the Immune Profile Score (IPS)—a powerful algorithm that provides prognostic insights into patient outcomes following treatment with immune checkpoint inhibitors (ICIs)—in ~18 months. This case study highlights the AI-driven methodology, real-world validation, and the impact of IPS in precision oncology.
Read more -
03/25/2025
AI & ML in action: Unlocking RWD with GenAI through Tempus Lens
Discover how Tempus is equipping researchers with innovative AI solutions to fully leverage the potential of multimodal data. Gain insights from a panel of leaders across healthcare and life sciences as they discuss the impact of these advanced tools on delivering insights with speed.
Watch replay
Secure your recording now. -
03/11/2025
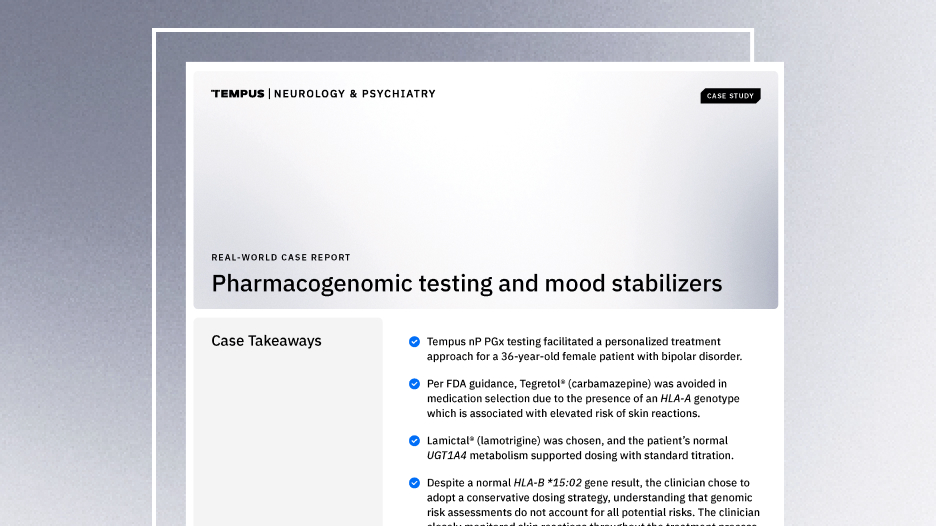
Case Report: Pharmacogenomic testing and mood stabilizers
This real-world case demonstrates how the Tempus nP pharmacogenomic test facilitated a personalized treatment approach for a patient with bipolar disorder.
Read more