-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
06/29/2024
Noninterventional Cohort Study of Biomarker Testing and Treatment Patterns in Patients With Advanced Gastric or Gastroesophageal Junction (G/GEJ) Cancer
ESMO GI 2024
PRESENTATION
Authors
S.J. Klempner, R. Fuldeore, A. Brackey, C. Sangli, S. Nishtala, D. Nimke, S. Braun, R. Mehta
Background – Combining biomarker-directed therapies with chemotherapy improves survival in patients with advanced G/GEJ cancer. Thus, timely testing for actionable biomarkers is needed to match patients with appropriate treatments, but data on real-world testing rates and treatment patterns are limited. This cohort study describes biomarker testing results and treatment patterns in patients with advanced G/GEJ cancer.
Methods – Deidentified health record data from the Tempus database in the US were used to identify adults with diagnosis codes for advanced G/GEJ cancer between 1 January 2020–11 July 2023. The primary outcomes were testing results for actionable biomarkers (human epidermal growth factor receptor 2 [HER2], programmed cell death ligand 1 [PD-L1], and microsatellite instability/mismatch repair [MSI/MMR]) and associated treatment patterns.
Results – The study included 829 patients with advanced G/GEJ cancer. Biomarker testing results and treatment patterns are reported in the table. Testing for all 3 biomarkers (HER2, PD-L1, and MSI/MMR) occurred in 363/829 (43.8%) patients in the Tempus database. Use of matched treatment based on actionable biomarkers occurred in only 107/826 (13%) patients.
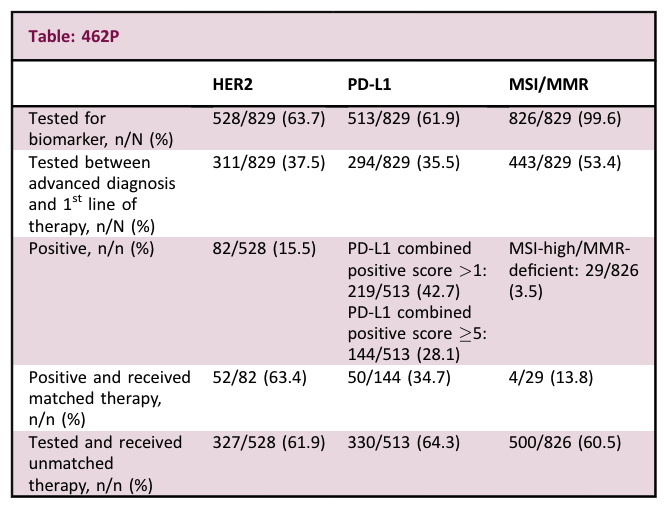
Conclusions – Contrary to current treatment guidelines, not all patients with advanced G/GEJ cancer had documentation of testing for actionable biomarkers, and of those who did, not all were treated with appropriate matched therapies. Increased biomarker testing and use of matched therapies would likely improve survival in these patients. Further research is needed to understand nonadherence to treatment guidelines.