-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
ENROLL NOW
Tempus’ patient-derived organoid screens
Evaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
09/12/2024
Q&A: Advancing immunotherapy biomarker identification with Immune Profile Score (IPS)
Jason Blue-Smith, VP & GM of Algos at Tempus, discusses the challenges in immunotherapy biomarker identification and how Tempus’ Immune Profile Score (IPS) offers solutions to these problems.
Authors
Jason Blue-Smith
Vice President & General Manager, Algos, Tempus

Vice President & General Manager, Algos, Tempus

As immunotherapy continues transforming cancer care, identifying the right patients for these therapies remains a significant hurdle. In this Q&A, Jason Blue-Smith, Vice President and General Manager of Algorithms at Tempus, explains how Tempus’ Immune Profile Score (IPS) is helping push the boundaries of biomarker development to overcome these challenges and improve patient outcomes.
Can you provide an overview of your role at Tempus? |
| Blue-Smith: I oversee the strategy and commercialization of algorithmic signatures developed both at Tempus and through external partnerships. Our teams assist life sciences companies in leveraging Tempus’ multimodal real-world data and biomarker development platforms to accelerate and advance their portfolios by generating actionable insights or producing the right data. |
What are the current challenges in immunotherapy biomarker identification? |
| Blue-Smith: Immunotherapy has revolutionized cancer treatment, delivering remarkable outcomes for many patients with previously untreatable cancers. However, there remains a significant opportunity to extend the benefits of immunotherapies to additional patients who are not currently identified by existing biomarkers.
The biology underlying each patient’s immune response is complex, and getting the right treatment to the right patients at the right time relies heavily on accurate biomarker identification. While current standard biomarkers like PD-L1 expression and tumor mutational burden (TMB) are widely used, they have limitations in predicting immune checkpoint inhibitor (ICI) response. For example, not all patients identified as PD-L1 or TMB-H respond to ICIs1, and conversely, some patients who could benefit from ICIs are not flagged by these biomarkers2-3. This inconsistency in predicting treatment outcomes highlights the need for more accurate and comprehensive biomarkers that can better capture the complexity of immune responses across different patient populations.
|
How do these challenges affect clinical trials and patient outcomes? |
| Blue-Smith: These challenges can contribute to high failure rates in clinical trials, where treatments may not perform as expected in the trial population. Addressing this issue requires a deeper understanding of the biology and action of investigational therapies from the earliest stages. This knowledge can improve patient selection, refine trial design, and isolate the mechanism of action, resulting in therapies that deliver stronger outcomes with lower toxicity. It all starts with an understanding of the biology and how different patient populations may respond. |
How does Tempus’ IPS algorithm address these challenges? |
| Blue-Smith: The next generation of cancer biomarker development is being driven by a multimodal, multi-omic data strategy that leverages AI on large, well-curated patient cohorts. In the case of IPS, we combined real-world data from genomic, transcriptomic, and clinical information from over 3,000 de-identified patient records and applied AI-assisted enhanced curation to build a comprehensive dataset for model development. Feature selection incorporated immune-inflammatory markers, immune resistance and tumor proliferation markers, immune checkpoint genes, and tumor-intrinsic features across a pan-cancer cohort in cancer types with ICI approvals.
Applying machine-learning techniques to this data resulted in what we now call IPS: a DNA and RNA-based molecular signature that demonstrates prognostic utility in an overall pan-cancer cohort of metastatic solid organ cancer patients. The output is a score that classifies study participants indicated for ICI treatment into one of two categories4:
|
In what ways can IPS support life sciences companies? |
Blue-Smith: There are three main use cases, in a research use only setting, where IPS can help accelerate progress for our life sciences partners:
IPS is available as an optional add-on for Tempus xT and xR tests ordered by life science partners and requires no additional tissue or wet lab assays. Results can be obtained from sequencing retrospective tissue samples or through flexible data licensing solutions that provide access to de-identified, multimodal patient records. Additionally, IPS can support companion diagnostic (CDx) development for both existing and emerging therapies.
|
What does the future hold for IPS? |
| Blue-Smith: This is just the beginning in terms of understanding and demonstrating the clinical benefit of IPS. Ongoing studies are further validating IPS’s ability to predict patient response in specific treatment settings, including novel combination therapies where patients may not respond well to approved ICI monotherapy. We recognize that many approaches and additional modalities, including digital pathology and clinicopathological data, will also be needed to fully assess a patient’s immune response. For instance, we’ve recently added a machine-learning-based algorithmic test, developed by Timothy A. Chan, MD, PhD, at Cleveland Clinic, to our immunotherapy biomarker portfolio. The test demonstrates an ability to predict the efficacy of immune checkpoint blockade based on patient-specific biological, laboratory, genomic, and clinical factors, complementing IPS.
Our ultimate goal is to bring these tests to clinical practice, where they can help physicians offer immunotherapy to more patients who are likely to benefit. |
To learn more about IPS, download our Immune Profile Score (IPS) Overview. For details on our clinical launch plans, please read our press release.
1. McGrail DJ, Pilié PG, Rashid NU, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661-672. doi:10.1016/j.annonc.2021.02.006
2. Rizvi H, Sanchez-Vega F, La K, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol. 2018;36(7):633-641. doi:10.1200/JCO.2017.75.3384. Correction in: J Clin Oncol. 2018 Jun 1;36(16):1645. doi:10.1200/JCO.2018.79.2796.
3. Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48. doi:10.1186/s40425-016-0153-x.
4. Some patients will have indeterminate results. This group was excluded from the validation study due to analytical precision concerns, and we cannot draw any conclusions about their outcomes.
-
11/11/2025
A new era of biopharma R&D: The TechBio revolution—realities and the next frontier
Join Tempus and Recursion leaders to explore their strategic TechBio partnership. Learn how they use AI and supercomputing with petabytes of data to accelerate drug discovery and development. See the impact on biopharma R&D's future.
Watch replay -
11/14/2025
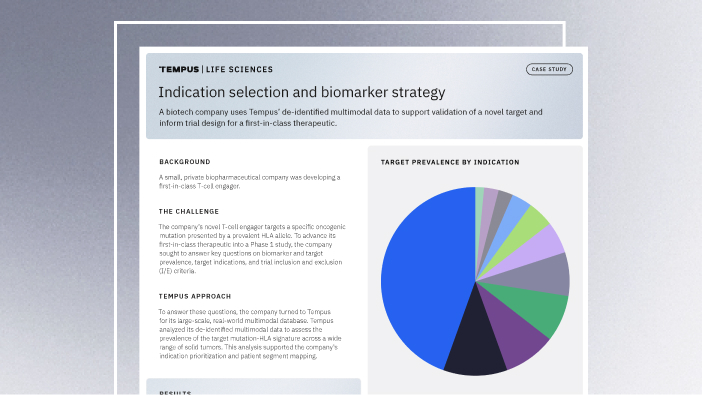
Validating a novel target and informing trial design for a first-in-class therapeutic
Discover how a biopharma company used Tempus’ de-identified multimodal data to support validation of a novel target and inform trial design for a first-in-class therapeutic.
Read more -
11/14/2025
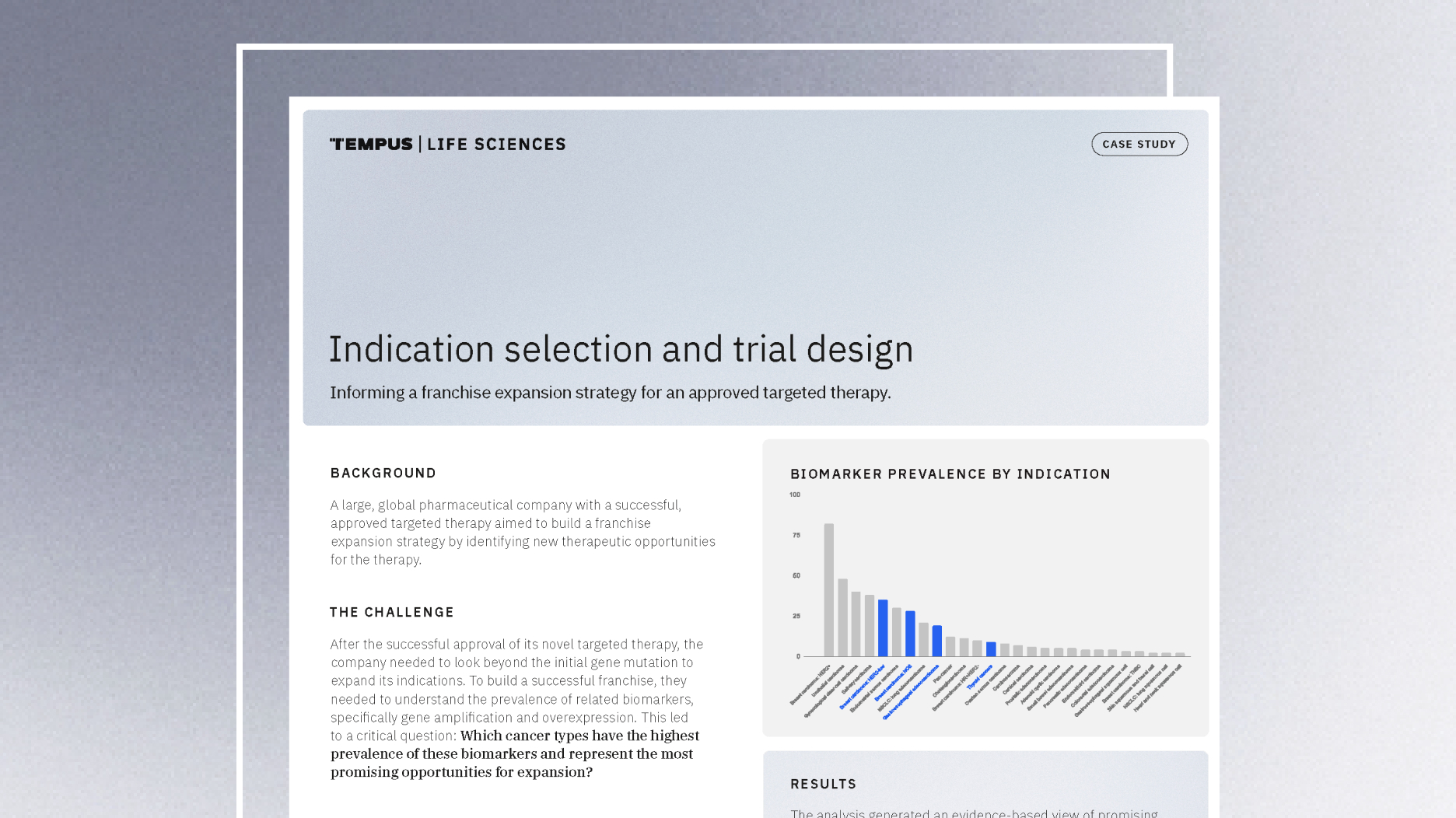
Guiding indication expansion with multimodal real-world data
Discover how a biopharma company used Tempus’ multimodal real-world data to guide its indication expansion strategy. See how our analysis of biomarker prevalence helped them identify new opportunities, prioritize R&D, and inform future trial design.
Read more