-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
11/05/2024
Variable Associations Between Humoral Immune Features And Immune Checkpoint Blockade-Related Outcomes Across Tissues In Metastatic Lung Adenocarcinoma
SITC 2024
PRESENTATION
Authors
Mario Rosasco, Rotem Ben-Shachar, Daniel Morgensztern, Michelle Stein, Justin Guinney
Background
Immune checkpoint blockade (ICB) has improved cancer patient outcomes, yet variable efficacy across metastatic sites motivates further study [1,2]. Recently, humoral immune features have emerged as ICB biomarkers [3]. Given strong correlations between many prognostic immune features [4], we evaluated heterogeneity in correlations between humoral and other features across metastatic sites, which may contribute to variable ICB efficacy.
Methods
De-identified RNAseq profiles from 1895 metastatic lung adenocarcinoma patients receiving any ICB-containing regimen in the first line of therapy were extracted from the Tempus Database. Samples were labeled by tissue origin: primary (n=695), neural (n=190), lymphatic (n=306), liver (n=124), or other (n=580). Immunological trait scores (e.g., overall infiltration, immune cell proportions) were computed using the Tempus IO platform [4]. Pearson correlations between traits were measured within each tissue, and differences were assessed using a z-test. In patients with a recorded death date or at least 2 years of follow-up (n=1125), features were tested for association with real-world overall survival (rwOS) at 2 years following metastatic diagnosis via a Wilcoxon rank-sum test with multiple testing correction using the Holm method.
Results
Patients with higher overall infiltration had higher rwOS at 2 years (37.25% vs. 28.32%, p=0.001). Across tissues, overall infiltration correlated with features such as a cytotoxicity signature (R=0.79) and expression of CD274 (R=0.39). Immune trait correlations in primary tissue were similar to lymphatic, but differed from neural, liver, and other metastases. Notably, correlations between plasma cells and overall infiltration in primary (R = 0.37) were significantly different from liver (R=0.09, p=0.003), neural (R=-0.20, p<0.001), and other tissues (R=0.09, p<0.001) (Figure 1). These differences persisted in samples stratified by CD274 expression. Features with differential correlations between tissues were associated with differences in rwOS. In primary tissue, higher cytotoxicity scores were significantly associated with rwOS (p=0.009) (Figure 2), and patients with higher cytotoxicity scores had higher rwOS at 2 years (40.31% vs. 25.12%, p=0.002). This association was also observed in neural (p=0.014), and other tissues (p=0.014). Conversely, plasma cell scores were only associated with rwOS in primary tissue (p=0.013). In primary tissue, patients with higher plasma cell scores had higher rwOS at 2 years (39.5% vs. 25.26%, p=0.003).
Conclusions
In a large real-world cohort of metastatic lung adenocarcinoma patients, we
observed significant immunological differences between tissues. Plasma cell
associations with rwOS varied between tissues, suggesting humoral immunity
may have tissue-dependent effects on ICB-related clinical outcomes.
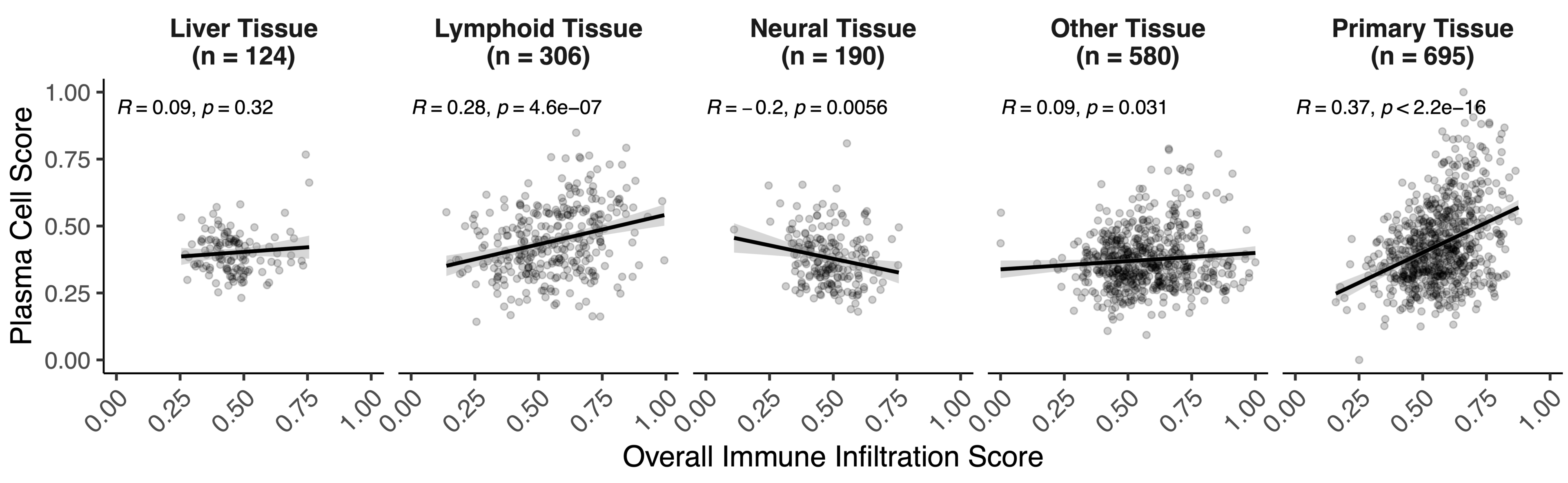
Figure 1 – Plasma cell correlations with overall immune infiltration differ by tissue. Z-tests were used to compare the correlation in primary tissue to correlations in liver (p=0.0026), lymphoid (p=0.1659), neural (p<0.001), and other tissues (p<0.001).
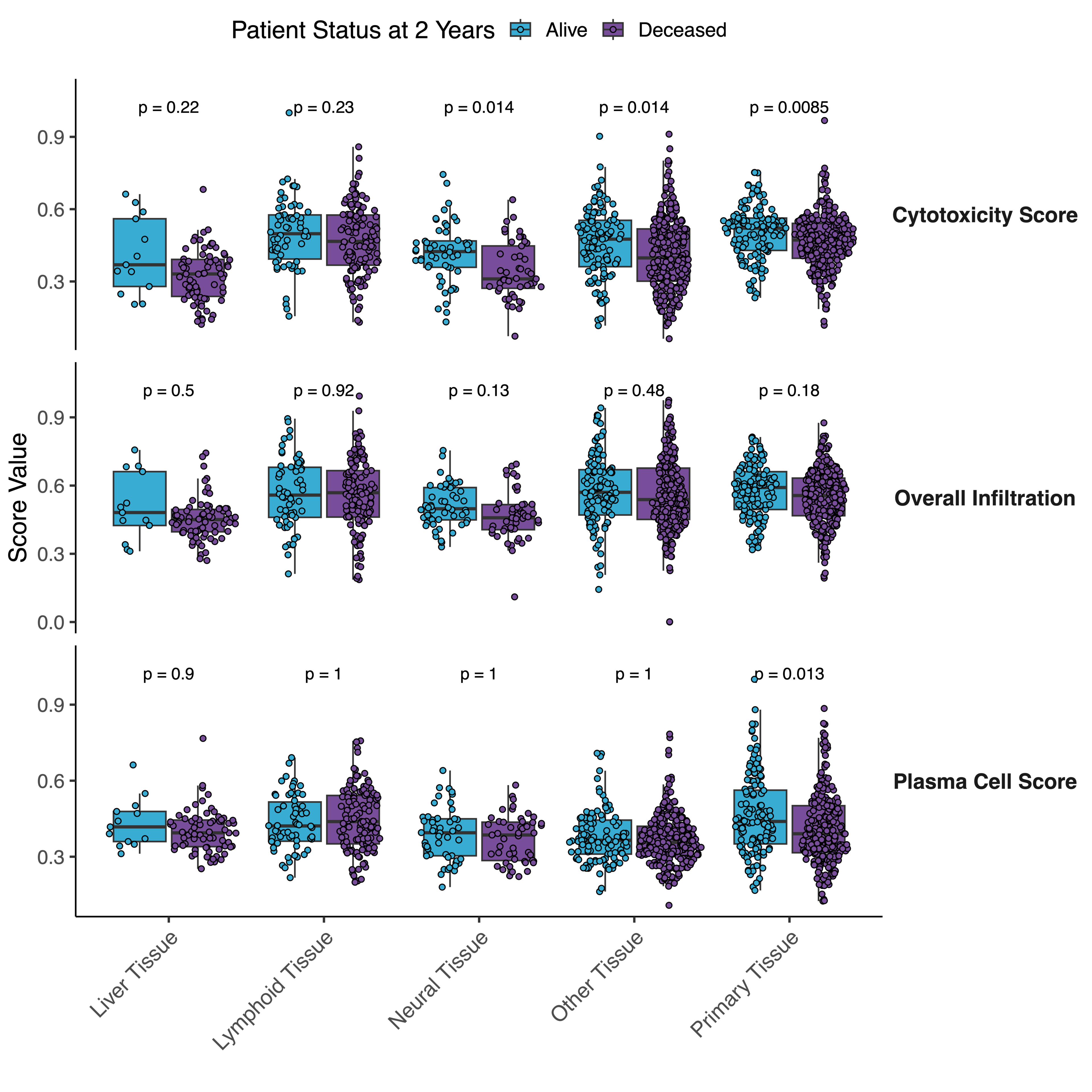 Figure 2 – Within each tissue, immune features are compared between patients who survive at 2 years following metastatic diagnosis (blue) and those who died within
Figure 2 – Within each tissue, immune features are compared between patients who survive at 2 years following metastatic diagnosis (blue) and those who died within
2 years following metastatic diagnosis (purple).
References
[1] Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, Davies MJ, Dubinett SM, Ferris A, Gandhi L, Garon EB, Hellmann MD, Hirsch FR, Malik S, Neal JW, Papadimitrakopoulou VA, Rimm DL, Schwartz LH, Sepesi B, Yeap BY, Rizvi NA, Herbst RS. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer. 2018 Jul 17;6(1):75. doi: 10.1186/s40425-018-0382-2. PMID: 30012210; PMCID: PMC6048854.
[2] Deng JY, Gou Q, Yang L, Chen ZH, Yang MY, Yang XR, Yan HH, Wei XW, Liu JQ, Su J, Zhong WZ, Xu CR, Wu YL, Zhou Q. Immune suppressive microenvironment in liver metastases contributes to organ-specific response of immunotherapy in advanced non-small cell lung cancer. J Immunother Cancer. 2023 Jul;11(7):e007218. doi: 10.1136/jitc-2023-007218. PMID: 37463790; PMCID: PMC10357800.
[3] Patil NS, Nabet BY, Müller S, Koeppen H, Zou W, Giltnane J, Au-Yeung A, Srivats S, Cheng JH, Takahashi C, de Almeida PE, Chitre AS, Grogan JL, Rangell L, Jayakar
S, Peterson M, Hsia AW, O’Gorman WE, Ballinger M, Banchereau R, Shames DS. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. 2022 Mar 14;40(3):289-300.e4. doi: 10.1016/j.ccell.2022.02.002. Epub 2022 Feb 24. PMID: 35216676.
[4] Stein MM, Rosasco M, Lau D, et al181 Associations between multimodal immune
biomarkers and clinical outcomes in a real-world non-small cell lung cancer cohort Journal for ImmunoTherapy of Cancer 2023;11:doi: 10.1136/jitc-2023-SITC2023.0181.