-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
11/08/2024
Q&A: Harnessing AI with multimodal data to transform drug development
Dr. Kate Sasser, PhD, Chief Scientific Officer at Tempus, discusses how Tempus layers ML and AI on multimodal data to drive insights and propel various stages of drug discovery and development.
Authors
Kate Sasser
PhD, Chief Scientific Officer at Tempus

PhD, Chief Scientific Officer at Tempus

As the advancement of AI continues transforming healthcare, life sciences companies are still determining how to fully leverage these technologies alongside precision medicine to enhance patient outcomes. In this Q&A, Kate Sasser, Chief Scientific Officer at Tempus, explains how Tempus is utilizing real-world data (RWD) for novel target discovery, clinical trial optimization, translational research, and evidence generation.
Can you provide a brief overview of Tempus as a precision medicine platform? |
| Sasser: The core of Tempus’ mission is to harness the power of technology, data, and artificial intelligence (AI) to enhance precision medicine, enabling physicians and drug developers to better match the right therapies to patients. Tempus is able to unlock precision medicine at scale, especially in oncology, since we have multiple products and services focused on this platform approach. Our multimodal dataset, now reaching over 250,000 patients with clinical, molecular, imaging, labs, and other types of data, can be leveraged with machine learning and AI methodologies to maximize insights. We combine this with intelligent diagnostics at scale, providing context to diagnostic assays and devices when they are woven into the broader ecosystem of the product suite. These diagnostics then feed back into the broader data model, providing a feedback loop that continues to strengthen our insight generation as we scale. We then make this data and insights available to the physicians and healthcare centers providing patient care, as well as life sciences companies developing the next generation of oncology medicine. |
How does AI help alleviate challenges throughout drug development? |
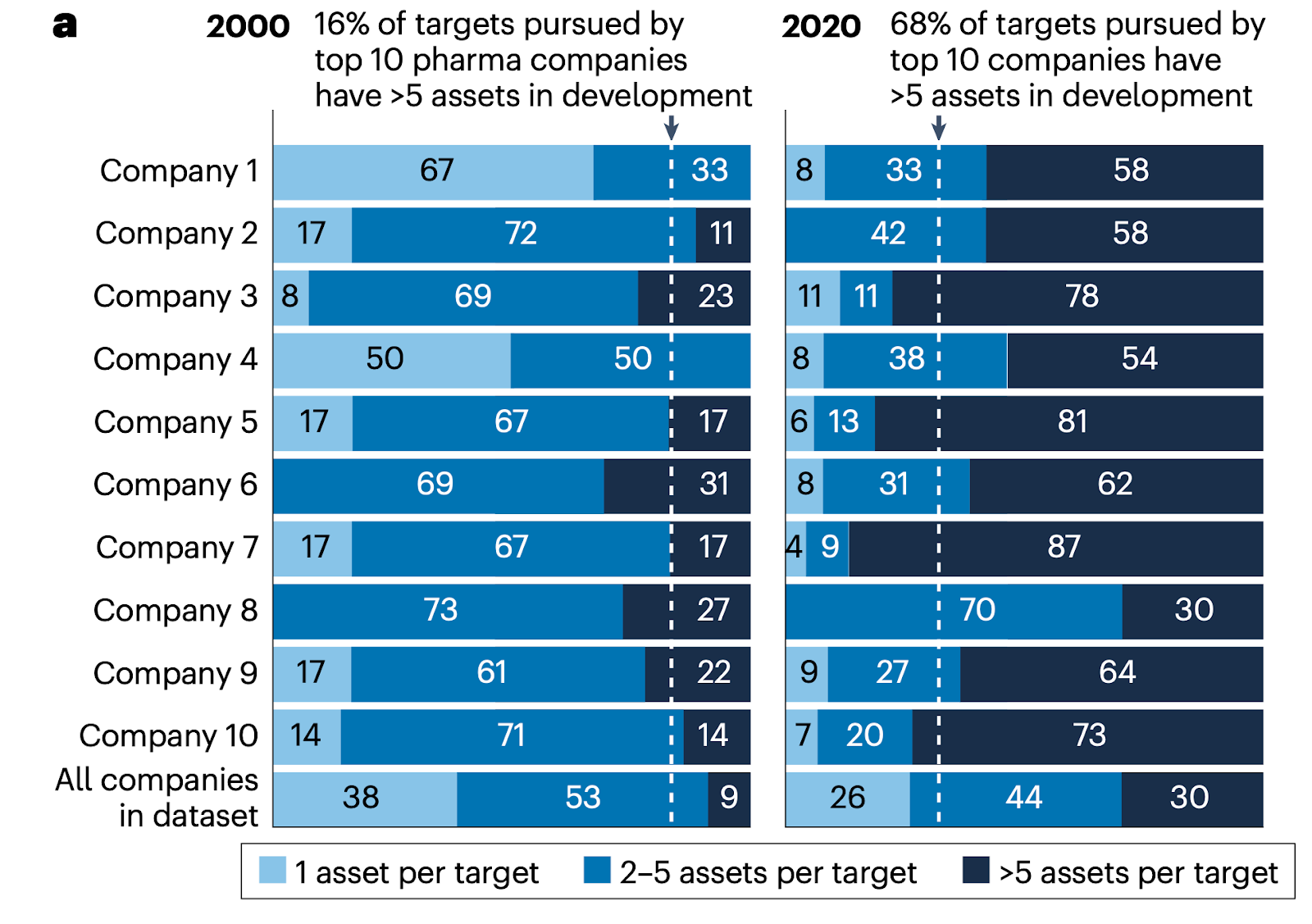
| Sasser: A major challenge in drug development is the lengthy timeline; it still takes approximately a decade from early target identification to getting a drug into the clinic. If we can leverage AI to reduce this timeframe to eight years, or perhaps even six, it would represent an incredible return on investment and exhibit a clear impact. Another challenge we are observing is diminishing returns in research and development (R&D) over time; life sciences companies are spending more but getting fewer drug approvals year after year. In 2023, there were only 55 FDA-approved drugs, with just 13 in oncology. Approvals have remained relatively flat despite increasing spending1. In oncology, there is significant competition and herding around certain targets. For example, for every new target entering the space, there are typically five or more assets in development targeting it. This highlights the challenge of identifying unique and innovative targets. Being able to identify novel targets is another area where we believe AI could ultimately play a significant role and start to demonstrate some initial value.
|
How is Tempus addressing these challenges through the application of RWD in drug discovery, development, and delivery? |
| Sasser: One of the areas within the discovery phase where Tempus believes AI and RWD are showing incredible promise for return on investment is in increasing novel target discovery. We’re supporting companies to achieve this by leveraging a key building block of our platform: the approximately seven million de-identified patient records that include genomic data, images, and clinical data. This is real-world, multimodal data which we combine with other tools and technologies, including knowledge graphs and feature embeddings, in order to ask unique questions of this data.
With this, we aim to not only speed up the process of identifying new targets but also enhance our ability to validate these targets and advance them through the development pipeline. When considering the platform in more depth, we are now able to utilize patient-derived organoids (PDO) within a lab-in-the-loop research ecosystem. This allows us to evaluate RWD insights alongside AI, machine learning tools, and other technologies to develop hypotheses, test those hypotheses in the organoid or wet lab setting, and then iterate in a more rapid fashion. Tempus also utilizes our real-world data and AI capabilities in early clinical development and translational research. This phase is often referred to as the “Valley of Death,” indicating that the likelihood of progressing from an early phase to a late phase study is exceedingly low. In light of this, Tempus aims to work closely with our life sciences customers to address three distinct areas:
One example of how we have successfully implemented this involved a life sciences company with a promising cell therapy application they wished to advance. Tempus harnessed our real-world data and developed an algorithm to identify human leukocyte antigen (HLA) loss of heterozygosity (LOH), and then again utilized RWD to understand the patient population and assist the company in identifying those patients in the real world. We assisted the company in refining their clinical trial and have helped them locate and enroll patients in the study. Similar to HLA LOH, we can examine other standard biomarkers like tumor mutational burden (TMB) or microsatellite instability (MSI), but we are also capable of developing novel biomarkers. We plan to present data at the Society for Immunotherapy of Cancer (SITC) this year on a novel immune signature called Immune Profile Score (IPS)3, which leverages different parameters, including a combination of DNA and RNA gene signatures, to identify patients who may likely to respond favorably to immune checkpoint inhibitors. We recognize that clinical trials are challenging and that fewer than 3% of patients enroll in a trial despite eligibility rates being much higher4. Tempus leverages its TIME network to identify patients who may be eligible for clinical trials and notify their treating physicians of the potential match. As we look ahead, Tempus is focused on helping to close care gaps. Physicians often struggle to keep abreast of novel therapies they could be utilizing, new guidelines, or new trials available. To address this, we have introduced a new feature that is yet another example of applying AI algorithms to our real-world data. Tempus Next can identify care gaps and promptly alert physicians or care teams, providing actionable insights to support access to the most up to date, guideline-directed treatments . For instance, it is a common misconception in the industry that every patient is receiving genomic sequencing, when in fact, this is not the case. This is a scenario where we can readily flag to physicians and healthcare systems when clinical guidelines recommend testing to help close that care gap. Finally, for companies aiming to develop new drugs or that already have drugs on the market, this data can be highly informative in terms of real-world utilization and the adoption of the precision medicine they are developing. In short, Tempus AI is constantly thinking about how to best utilize our continuously growing real-world dataset. We explore new ways to apply machine learning, AI, and novel algorithms alongside delivery mechanisms, to help deliver precision medicine that positively impacts patient care. |
1. Center for Drug Evaluation and Research. Novel drug approvals for 2023. U.S. Food and Drug Administration. Published October 11, 2024. https://www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2023. doi:10.1016/j.annonc.2021.02.006
2. Fougner C, et al. Herding in the drug development pipeline. Nat Rev Drug Discov. 2023;22:617-618. doi:10.1038/d41573-023-00063-3.
3. At the time of publication, Immune Profile Score (IPS) is exclusively available for life science partners for research use only. To learn more, click here.
4. Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728-1733.
-
04/02/2025
Development of a clinical algorithm to prognosticate response to immunotherapy
Discover how Tempus developed and deployed the Immune Profile Score (IPS)—a powerful algorithm that provides prognostic insights into patient outcomes following treatment with immune checkpoint inhibitors (ICIs)—in ~18 months. This case study highlights the AI-driven methodology, real-world validation, and the impact of IPS in precision oncology.
Read more -
03/25/2025
AI & ML in action: Unlocking RWD with GenAI through Tempus Lens
Discover how Tempus is equipping researchers with innovative AI solutions to fully leverage the potential of multimodal data. Gain insights from a panel of leaders across healthcare and life sciences as they discuss the impact of these advanced tools on delivering insights with speed.
Watch replay
Secure your recording now. -
03/11/2025
Case Report: Pharmacogenomic testing and mood stabilizers
This real-world case demonstrates how the Tempus nP pharmacogenomic test facilitated a personalized treatment approach for a patient with bipolar disorder.
Read more
 2
2