-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
ENROLL NOW
Tempus’ patient-derived organoid screens
Evaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
11/05/2024
BASECAMP-1 Is an Efficient Pre-Screening Study That Identifies Patients With HLA LOH and Provides Mutational, RNA-Seq, and Microbiome Data for Precision Logic-Gated Car T Therapeutic Trials
SITC 2024
PRESENTATION
Authors
Julian R Molina, Diane M Simeone, Kristen Spencer, Patrick M Grierson, Matthew Ulrickson, Kedar Kirtane, Maria Pia Morelli, Jennifer Specht, Saurabh Dahiya, Hemant S Murthy, Jong Chul Park, William Y Go, Sandip Pravin Patel, Armen Mardiros, Marwan G Fakih, Jessica Tebbets, Marco Davila, Kirstin Liechty, Marcela Maus, Eric W Ng, David G Maloney, John Welch and J Randolph Hecht
Background Precision medicine studies must overcome the operational burden of prescreening patients and small cohort sizes for correlative studies. BASECAMP-1 (NCT04981119) is an ongoing prescreening study that uses a single next-generation sequencing assay (Tempus AI Inc) to address both problems by: 1) efficiently screening tumor-associated human leukocyte antigen (HLA)-A*02 loss of heterozygosity (LOH) for subsequent trials of TmodTM logic-gated chimeric antigen receptor T-cell (CAR T) therapy1 2 (eg, EVEREST-1 [NCT05736731], EVEREST-2 [NCT06051695]3 4; and 2) providing a large dataset available for translational studies to augment statistical power of discovery analyses.
Methods Patients are screened using the Tempus xT platform, which analyzes tumor tissue for HLA-A LOH, gene mutations, and RNA sequencing (RNA-Seq). Once enrolled, eligible patients undergo leukapheresis in anticipation of therapeutic studies. Patients may be identified through AWARE, which informs principal investigators of Tempus-sequenced patients within their health system who match the key eligibility criteria for BASECAMP-1 (figure 1). Enrolled patients with colorectal (CRC), non-small cell lung (NSCLC), ovarian (OVCA), and pancreatic cancer (PANC) were analyzed using xT results for tumor purity, mutation distribution, HLA-A*02 LOH status, and RNA-Seq.
Results From November 2021 to June 1, 2024, 1464 patients were consented for BASECAMP-1 across 13 institutions (figure 2). HLA status was determined for 1422 patients; 63 (4.4%) were HLA-A*02 LOH positive, of which 31 have undergone leukapheresis. Since April 2023, AWARE has increased the patient identification rate from 0.6 to 3.4 HLA-A*02 LOH patients identified/month. Correlative data from xT were available for 318 patients with germline HLA-A*02. Tumor purity was lower in PANC and higher in OVCA specimens vs CRC and NSCLC, providing insight into projected screening success rates based on LOH assay sensitivity thresholds. Mutation distribution was as expected, although TP53 and LRPB1 were more common in NSCLC with LOH than without (table 1). LOH status was not associated with tumor-specific differences. In RNA-Seq, CEACAM5 was expressed higher in CRC and MSLN in PANC and OVCA. RNA-Seq-imputed immune cell infiltrates were higher in NSCLC. Microbiome RNA-Seq-based analysis identified Acinetobacter specifically in CRC, as expected, and Mesorhizobium, Paeniclostridium, Delftia, and Afipia in PANC.
Conclusions Identification and enrollment of HLA-A*02 LOH patients into BASECAMP-1 for logic-gated CAR T therapeutic studies was accelerated by AWARE. Concurrent xT analysis demonstrated expected results in tumor purity, mutation distribution, target expression, and microbiome. The large volume of data provides opportunities to augment analyses from therapeutic trials, including robust propensity-matched comparisons, response correlation studies, and other translational discovery.
Acknowledgements The authors would like to thank the following: Patients and their families and caregivers for participating in the study, the screeners, clinical research coordinators, study nurses, data managers, and apheresis teams at all of the study sites, and contributions from others at A2 Bio. Medical writing support was provided by Bio Connections, LLC, and funded by A2 Bio.
Trial Registration NCT04981119.
References
-
Hamburger AE, DiAndreth B, Cui J, et al. Engineered T cells directed at tumors with defined allelic loss. Mol Immunol 2020;128:298–310.
-
DiAndreth B, Hamburger A, Xu H, Kamb A. The Tmod cellular logic gate as a solution for tumor-selective immunotherapy. Clin Immunol 2022;241:109030.
-
Sandberg ML, Wang X, Martin AD, et al. A carcinoembryonic antigen-specific cell therapy selectively targets tumor cells with HLA loss of heterozygosity in vitro and in vivo. Sci Transl Med 2022;14:eabm0306.
-
Tokatlian T, Asuelime GE, Mock J-Y, et al. Mesothelin-specific CAR-T cell therapy that incorporates an HLA-gated safety mechanism selectively kills tumor cells. J Immunother Cancer 2022;10:e003826.
Ethics Approval This study was approved by site IRBs.
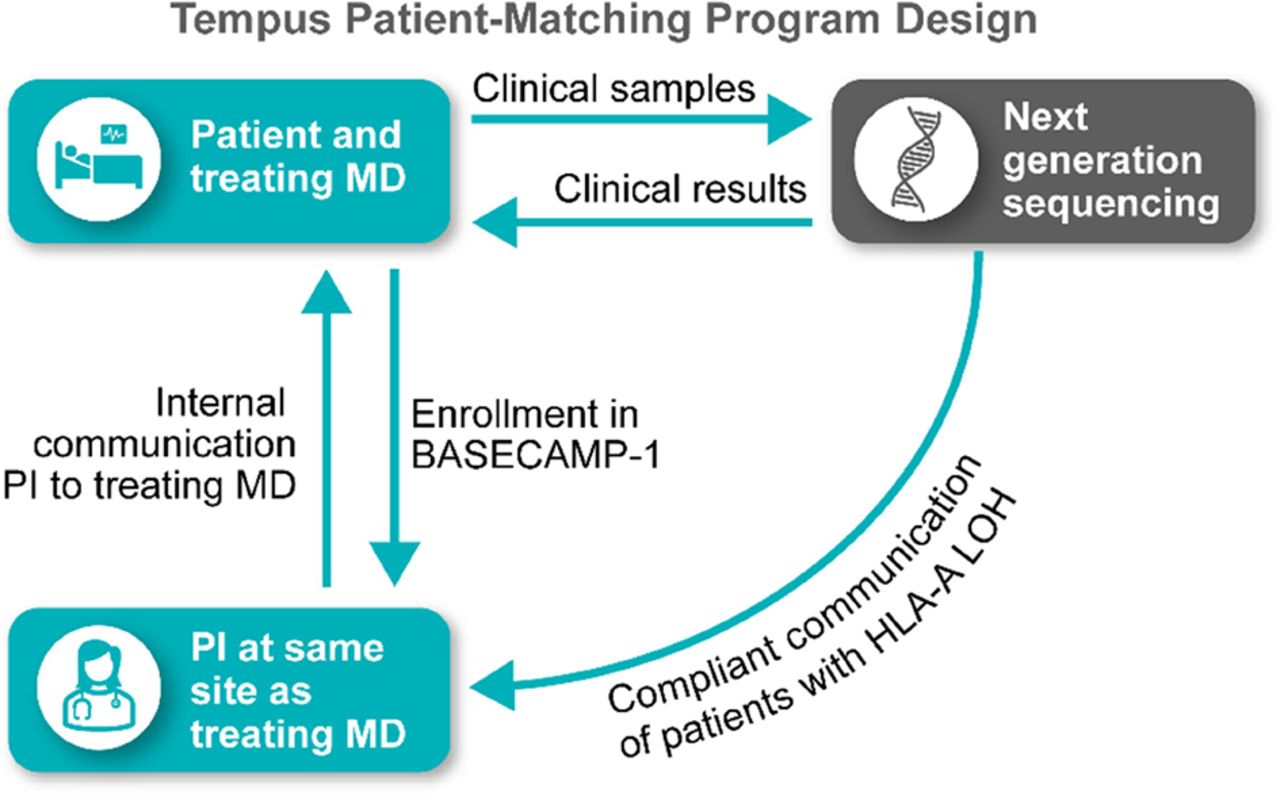
Tempus patient-matching program identifies eligible patients for BASECAMP-1. PI, principal investigator
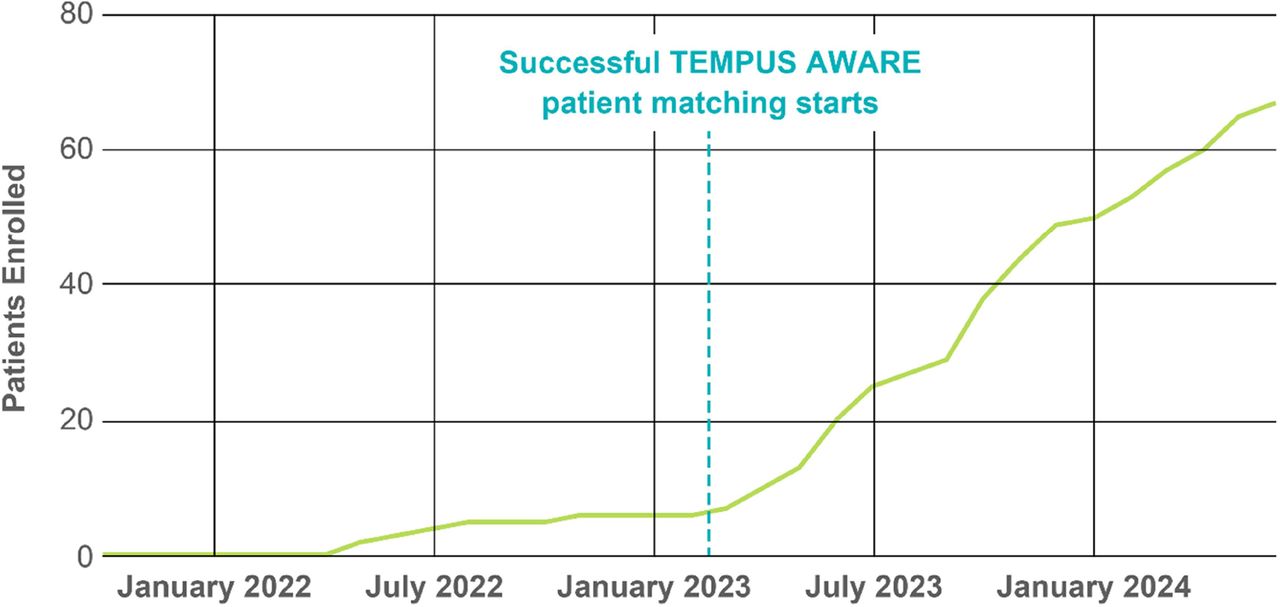 Patients in BASECAMP-1 identified with HLA-A*02 LOH since 2021
Patients in BASECAMP-1 identified with HLA-A*02 LOH since 2021

Tumor mutation distribution by cancer type among patients with HLA-A*02 LOH in BASECAMP-1a TP53 and LRPB1 were more common in patients with NSCLC with HLA-A*02 LOH than without (chi-squared < 0.05). This table includes patients enrolled in BASECAMP-1 with germline HLA-A*02 LOH status and correlative data available. Data as of 01 June 2024