-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
Tempus Cardiology
AI-enabled solutions to help providers close care gaps for patients who may have undiagnosed and undertreated cardiovascular disease.
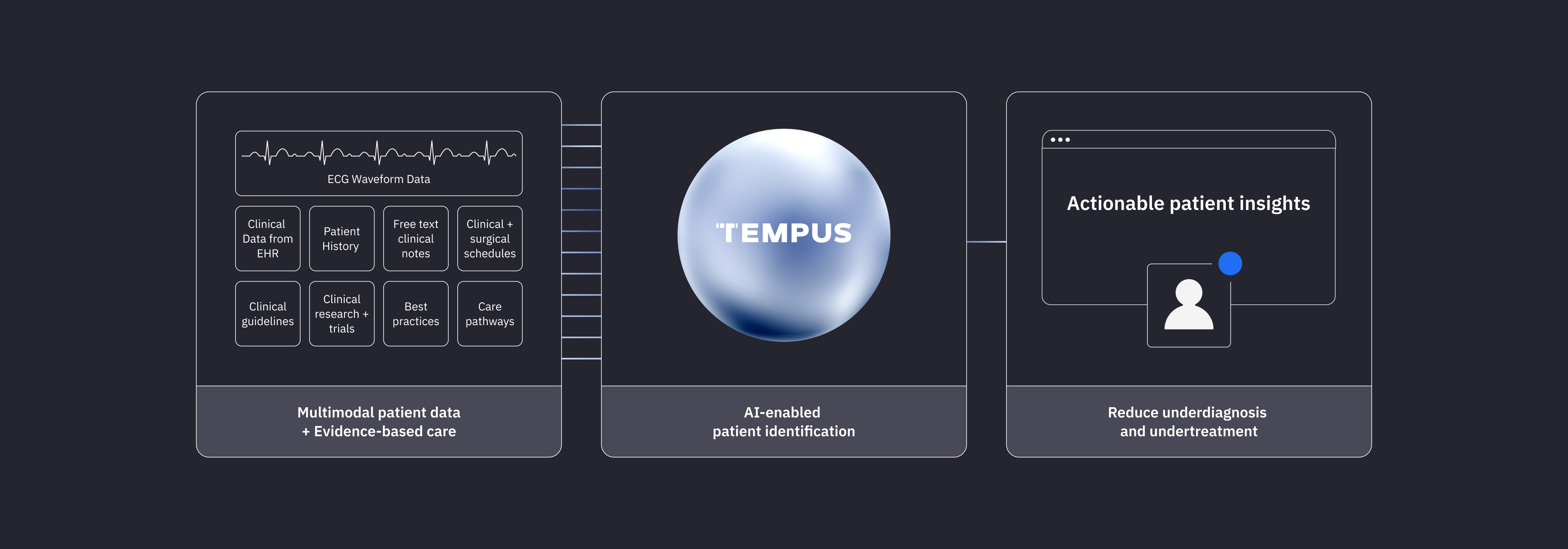
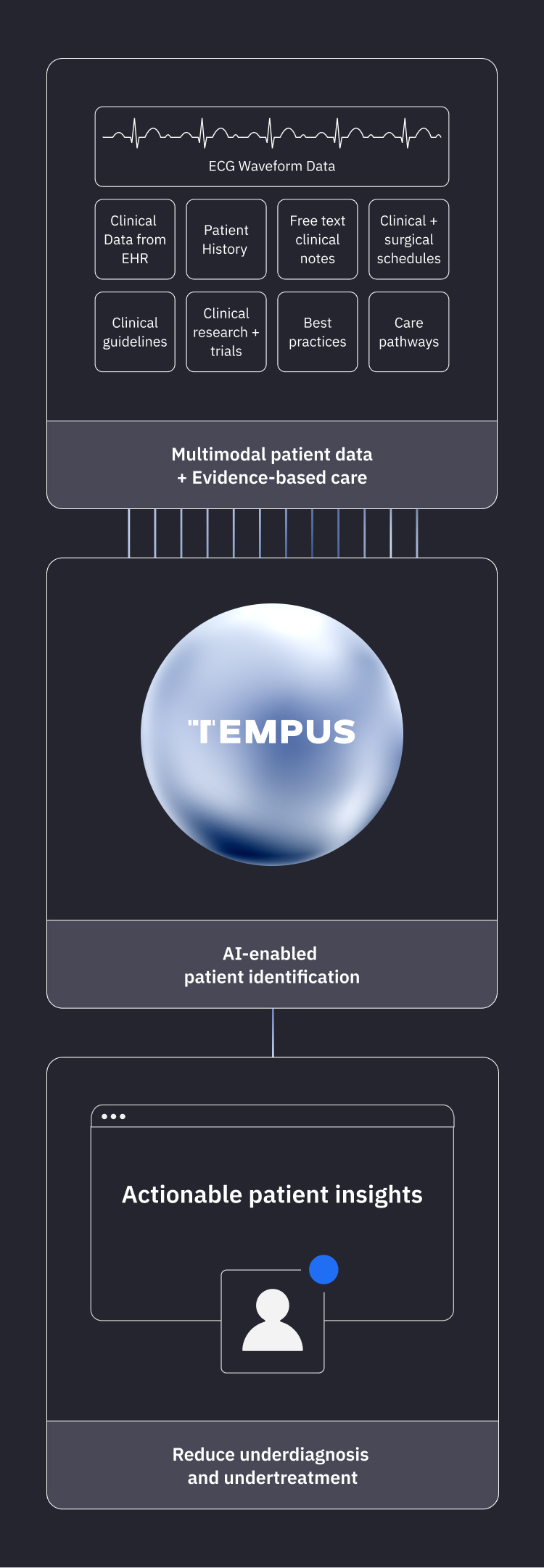
Our Impact
-
60+
algorithms to identify potential care gaps across 15 cardiovascular diseases
-
~2M
patients screened
-
50K+
patients screened per month
-
100+
hospitals nationwide are currently powered by Tempus Next

Solutions to help identify your patients
Underdiagnosed patients
Tempus accelerates cardiac care with AI-powered solutions to identify potentially undiagnosed patients.
ECG-AI
ECG-AI devices integrate with advanced cardiology algorithms to detect signs of disease and notify care teams for patient follow up.
Learn morePixel for Radiology
Advanced viewing and automated reporting of cardiac MR images help improve efficiency and accuracy in flow visualization and quantification, functional analysis, and tissue characterization.
Learn moreUndertreated patients
Tempus empowers clinicians to deliver the next step in a patient’s care journey with our AI-enabled care pathway intelligence platform.
Tempus Next
60+ algorithms to identify potential care gaps across 15 cardiovascular diseases including aortic stenosis, mitral regurgitation, congestive heart failure, abdominal aortic aneurysms and more.
Learn more-
UPCOMING WEBINAR:
Tempus Receives U.S. FDA 510(k) Clearance for Tempus ECG-AF, an AI-based Algorithm that Identifies Patients at Increased Risk of AFib
Tempus AI, Inc. (NASDAQ: TEM), a leader in artificial intelligence and precision medicine, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Tempus ECG-AF device that uses AI to help identify patients who may be at increased risk of atrial fibrillation/flutter (AF).
Link -
UPCOMING WEBINAR:
New CMS Decision Provides Medicare Coverage for the Clinical Use of the Tempus ECG-AF Device
Tempus AI, Inc. (NASDAQ: TEM), a technology company leading the adoption of AI to advance precision medicine and patient care, today announced the impact of a new decision by the Centers for Medicare and Medicaid Services (CMS) that will allow reimbursement for assessments of cardiac dysfunction using the Tempus ECG-AF algorithm.
Link -
UPCOMING WEBINAR:
Tempus and Northwestern Medicine Announce Collaboration to Bring Practical Application of AI to Healthcare
Tempus, a leader in artificial intelligence and precision medicine, and Northwestern Medicine, Chicago’s premier integrated academic health system, today announced a collaboration that aims to explore the application of artificial intelligence in clinical care and research.
Link -
UPCOMING WEBINAR:
Tempus Announces Collaboration with United Therapeutics to Study Use of AI to Detect Patients at Risk for Pulmonary Hypertension
Tempus AI, Inc. (NASDAQ: TEM), a leader in artificial intelligence and precision medicine, today announced a new collaboration with United Therapeutics (UT), a leading biotechnology company focused on providing a brighter future for patients through the development of novel pharmaceuticals and technologies.
Link
Our Science
view all-
UPCOMING WEBINAR:
A Novel Phenotyping Pipeline to Improve Identification of Patients with Pulmonary Hypertension in Electronic Health Records
05/22/2024
Read more -
UPCOMING WEBINAR:
ECG-based Machine Learning Model Identifies Patients at High Risk for Incident Pulmonary Hypertension
05/22/2024
Read more -
UPCOMING WEBINAR:
Structured EHR Data Underestimates Prevalence and Misses Large Proportions of Patients with Pulmonary Hypertension
05/22/2024
Read more -
UPCOMING WEBINAR:
Predictors of Disease Progression and Adverse Clinical Outcomes in Patients With Moderate Aortic Stenosis Using an Artificial Intelligence-Based Software Platform
05/04/2024
Read more -
UPCOMING WEBINAR:
Large Language Models with Retrieval-Augmented Generation for Zero-Shot Disease Phenotyping
12/11/2023
Read more
Partnering with Tempus is investing in the future
We believe that AI-enabled solutions can help shorten the diagnostic odyssey and improve the lives and outcomes of patients with cardiovascular disease.