-
PROVIDERS
READ NOW
Minimal residual disease (MRD) testing: A practical guide for clinicians
-
LIFE SCIENCES
READ NOW
Q&A: Harnessing generative AI to refine clinical research & drug development
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS

Providers /// Dermatology
Precision insights for enhanced melanoma patient care
Refine clinical decisions with an algorithmic tool for early-stage melanoma risk assessment and management.
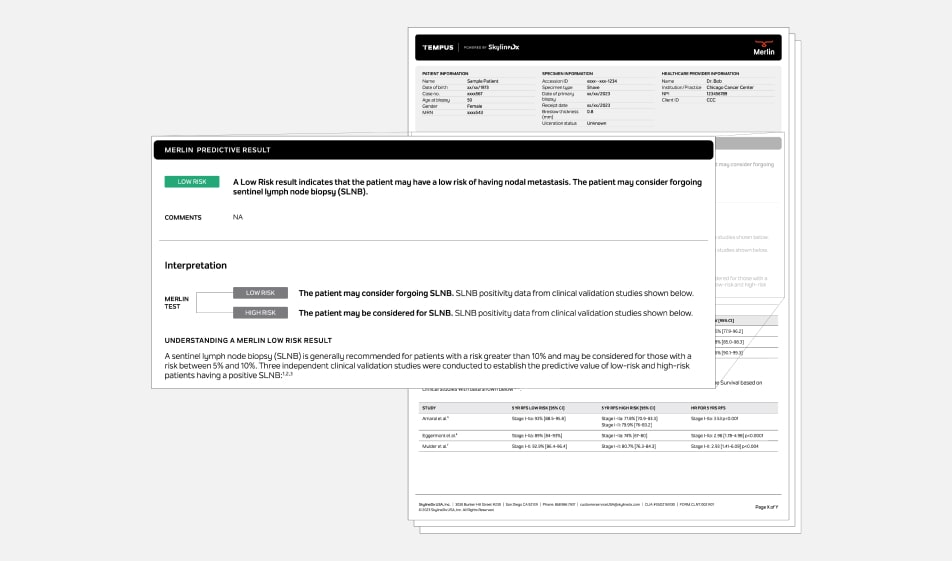
Tempus Merlin (powered by SkylineDx)* informs management of early-stage melanoma patients
Merlin leverages a combination of clinicopathologic features with gene expression profiling components (CP-GEP) to provide a risk assessment that can help inform key clinical management decisions.
*Tempus Merlin was developed and is performed by Skyline Dx as a reference laboratory.
Actionable results
Merlin provides an easy-to-interpret binary result that informs both the risk of recurrence and the likelihood of sentinel lymph node biopsy (SLNB) positivity, to help inform physicians on the intensity of follow-up and whether the patient may consider forgoing the SLNB procedure.
Validation Data
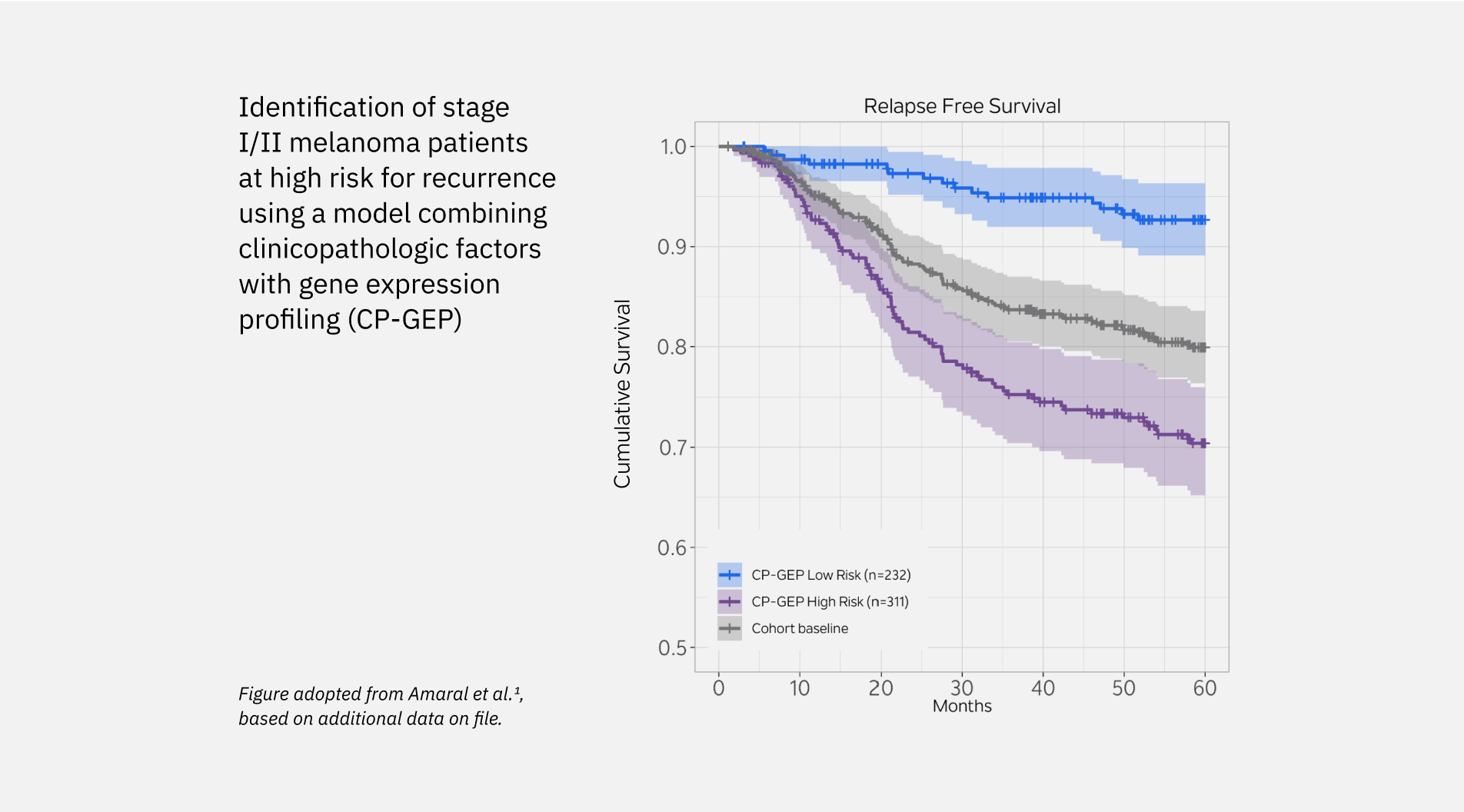
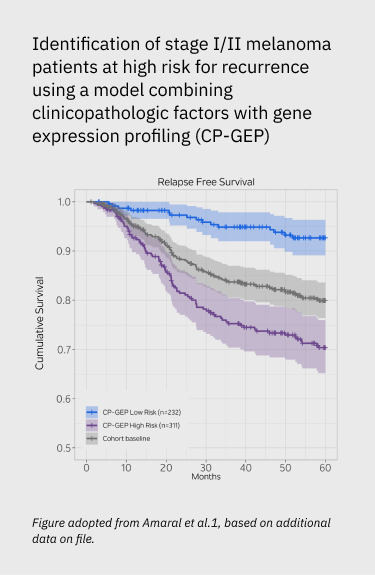
Merlin has been validated in multiple independent studies, across diverse patient populations, with both retrospective and prospective data.- Merlin Low Risk patients were roughly 3x less likely to relapse over a 5 year period than Merlin High Risk in stage I-II patients.1-3
- Merlin Low Risk patients have a better prognosis than Merlin High Risk patients (93% group avg survival versus 77.8% group avg survival over 60 months for stage la-ll patients).1
- The Merlin test classified 38.5% of patients as low risk, with a 96.7% NPV amongst T1-T4 patients (n=222).4
-
Algorithmic Accuracy
24% of T1-T3 patients were classified as low risk in the largest validation study for Merlin. 96.5% NPV was achieved, with 83 out of 86 low risk patients subsequently exhibiting negative SLNB.5
-
Ease of Use
Easy to interpret binary results stratify patients as Low Risk or High Risk. The Tempus Hub allows for fast and secure access to Merlin orders and results.
-
Turnaround Time
Merlin results are delivered within 5 days of sample receipt.
-
Tissue Requirements
5 x 10 microns formalin fixed and paraffin embedded (FFPE) tissue of primary cutaneous melanoma biopsy (via fresh cut curls, slides, or a block); no macrodissection is needed.
-
Additional Testing
The Tempus platform can be leveraged throughout the patient’s journey offering additional solutions for late stage melanoma patients, including Tempus xT tumor profiling and xF liquid biopsy
24% of T1-T3 patients were classified as low risk in the largest validation study for Merlin. 96.5% NPV was achieved, with 83 out of 86 low risk patients subsequently exhibiting negative SLNB.5
Easy to interpret binary results stratify patients as Low Risk or High Risk. The Tempus Hub allows for fast and secure access to Merlin orders and results.
5 x 10 microns formalin fixed and paraffin embedded (FFPE) tissue of primary cutaneous melanoma biopsy (via fresh cut curls, slides, or a block); no macrodissection is needed.
The Tempus platform can be leveraged throughout the patient’s journey offering additional solutions for late stage melanoma patients, including Tempus xT tumor profiling and xF liquid biopsy
Merlin Test Indications
Tempus Merlin is intended for T1a-T3 melanoma patients eligible for an SLNB
- Newly diagnosed invasive malignant melanoma of skin (AJCC 8th edition staging guidelines).
- Without clinical evidence of nodal involvement or distant metastasis (cN0M0).
- T1a lesions with Breslow thickness >0.5mm and other adverse features (age ≤42 years, head/neck location, lymphovascular invasion, and/or mitotic rate ≥2/mm2).
- T1b-T3 (Breslow thickness ≥0.8mm to 4.0mm or <0.8mm with ulceration).
Financial Assistance
We help provide access to our tests for all patients in financial need
Approval of the financial assistance application is based on your household income and takes into account all life circumstances. Once a financial assistance application is submitted either online or over the phone, you will receive a decision at the time of submission.
-
Step 1
Apply for financial assistance online at access.tempus.com.
-
Step 2
If approved, you will know immediately about the maximum out-of-pocket cost of your testing.
-
Step 3
Please contact billing@tempus.com if you are concerned about out-of-pocket costs and would like to discuss your options.
All U.S.-based patients are eligible to apply for financial assistance regardless of insurance status. For uninsured and international patients, we offer a self-pay option. If you have any questions, please email patients@tempus.com.
-
UPCOMING WEBINAR:
Identification of stage I/II melanoma patients at high risk for recurrence using a model combining clinicopathologic factors with gene expression profiling (CP-GEP)
Read more -
UPCOMING WEBINAR:
Clinical Evidence of the clinicopathologic and gene expression profile (CP-GEP) in patients with melanoma eligible for sentinel lymph node biopsy: A multicenter prospective Dutch study
Read more -
UPCOMING WEBINAR:
Validation of a clinicopathological and gene expression profile model to identify patients with cutaneous melanoma where sentinel lymph node biopsy is unnecessary
Read more
- Amaral T, Sinnberg T, Chatziioannou E, et al. Identification of stage I/II melanoma patients at high risk for recurrence using a model combining clinicopathologic factors with gene expression profiling (CP-GEP). Eur J Cancer. 2023;182:155-162.
- Eggermont AMM, Bellomo D, Arias-Mejias SM, et al. Identification of stage I/IIA melanoma patients at high risk for disease relapse using a clinicopathologic and gene expression model. Eur J Cancer. 2020;140:11-18.
- Mulder EEAP, Johansson I, Grünhagen DJ, et al. Using a clinicopathologic and gene expression (CP-GEP) model to identify stage I-II melanoma patients at risk of disease relapse. Cancers (Basel). 2022;14(12):2854.
- Stassen RC, Mulder EEAP, Mooyaart AL, et al. Clinical Evidence of the clinicopathologic and gene expression profile (CP-GEP) in patients with melanoma eligible for sentinel lymph node biopsy: A multicenter prospective Dutch study. Eur J Surg Oncol. 2023; 49(12):107249.
- Johansson I, Tempel D, Dwarkasing JT, et al. Validation of a clinicopathological and gene expression profile model to identify patients with cutaneous melanoma where sentinel lymph node biopsy is unnecessary. Eur J Surg Oncol. 2022;48(2):320-325.
This is data-driven precision medicine
This is the future of healthcare.