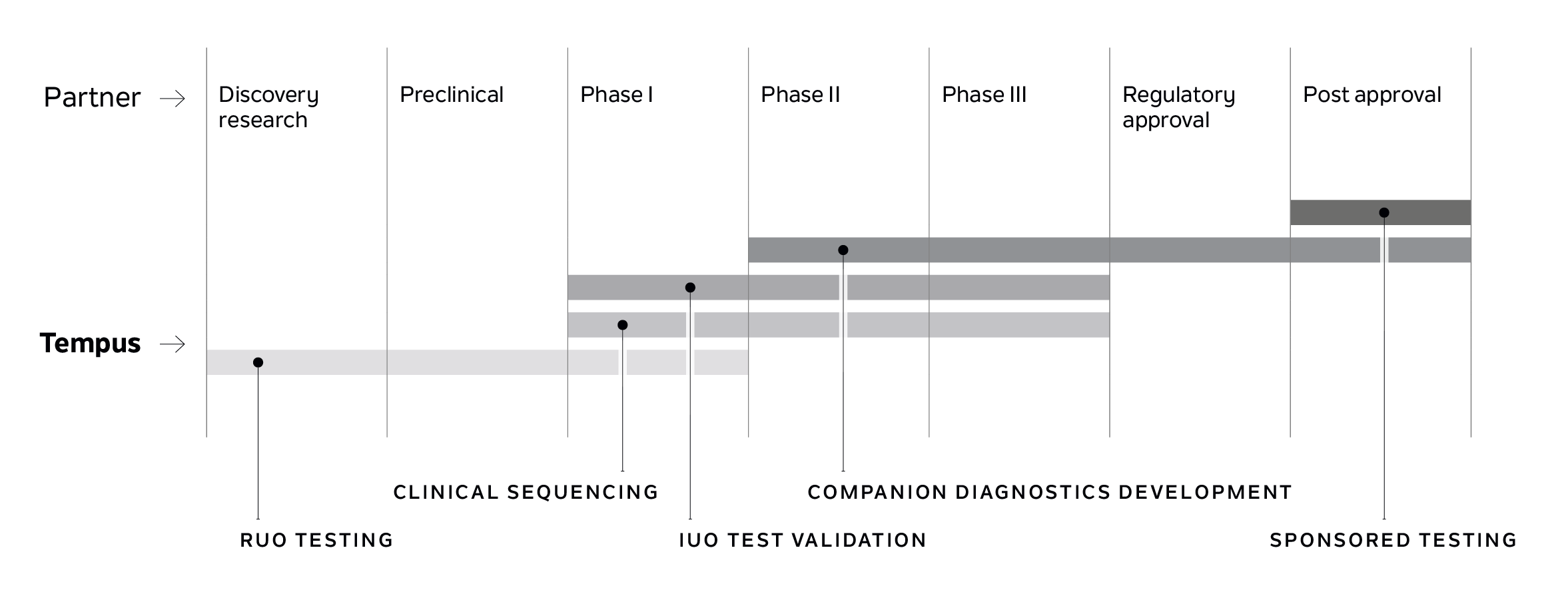
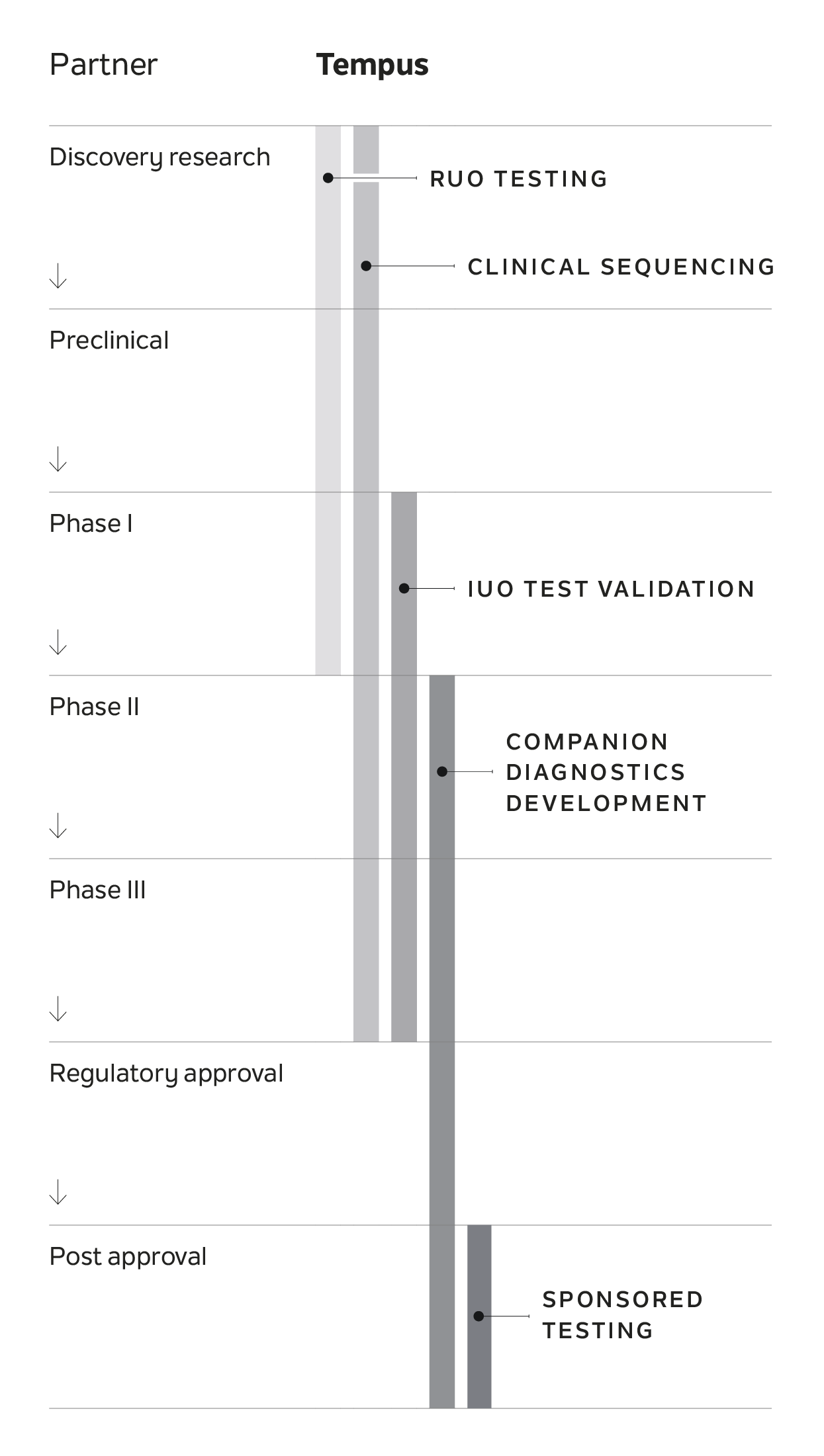
Tempus IO™
A comprehensive immunotherapy platform that provides actionable and cutting-edge services for life sciences partners advancing IO therapies, including antibody-drug conjugates (ADCs), TCR therapies, and immune checkpoint inhibitors (ICIs).
Learn more