-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
 Portfolio of Minimal Residual Disease & Monitoring
Portfolio of Minimal Residual Disease & Monitoring
 Portfolio of Minimal Residual Disease & Monitoring
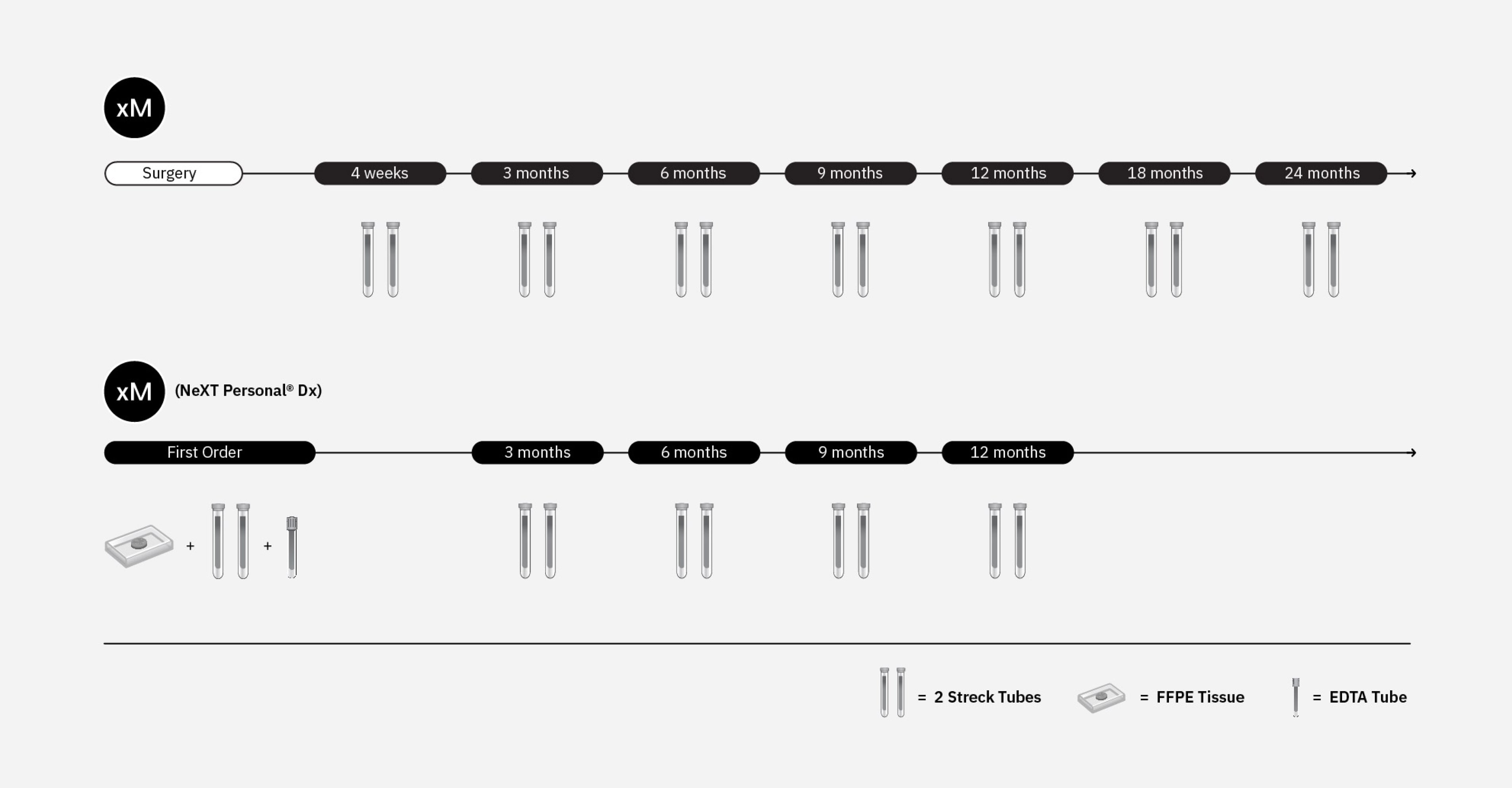
Portfolio of Minimal Residual Disease & MonitoringOur portfolio of tumor-naive and tumor-informed minimal residual disease (MRD) and monitoring assays provides physicians with valuable patient insights to individualize treatment plans.
xM is a finely tuned MRD assay that delivers rapid results from one blood draw to help detect residual disease or recurrence in colorectal cancer.
xM (NeXT Personal® Dx)* is an ultra sensitive, whole genome assay that enables detection of ctDNA at very low levels to monitor residual disease and recurrence and IO treatment response monitoring.
*Test by Personalis
xM is a tumor-naive, finely tuned assay for colorectal cancer

xM (NeXT Personal® Dx) is a tumor-informed, ultra-sensitive whole genome assay for solid tumors
Detection of ctDNA may function as an early indicator for recurrent malignancy, prior to being seen on radiographic scans.
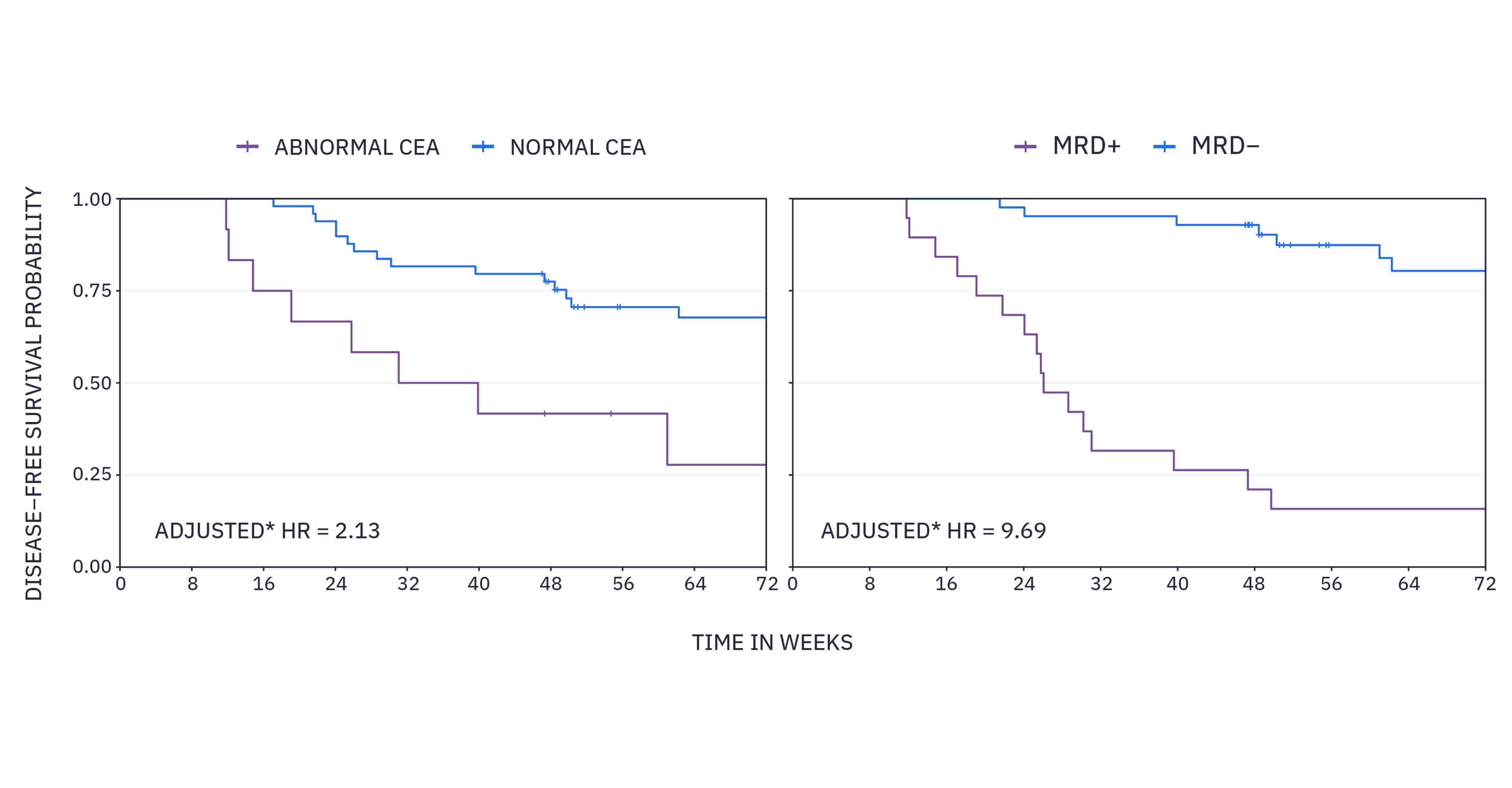
xM CLINICAL PERFORMANCE IN COLORECTAL CANCER 2
From a subset analysis from the GALAXY study in CIRCULATE-Japan, xM results predict disease-free survival (DFS) nearly 5 times superior to standard of care carcinoembryonic antigen (CEA).
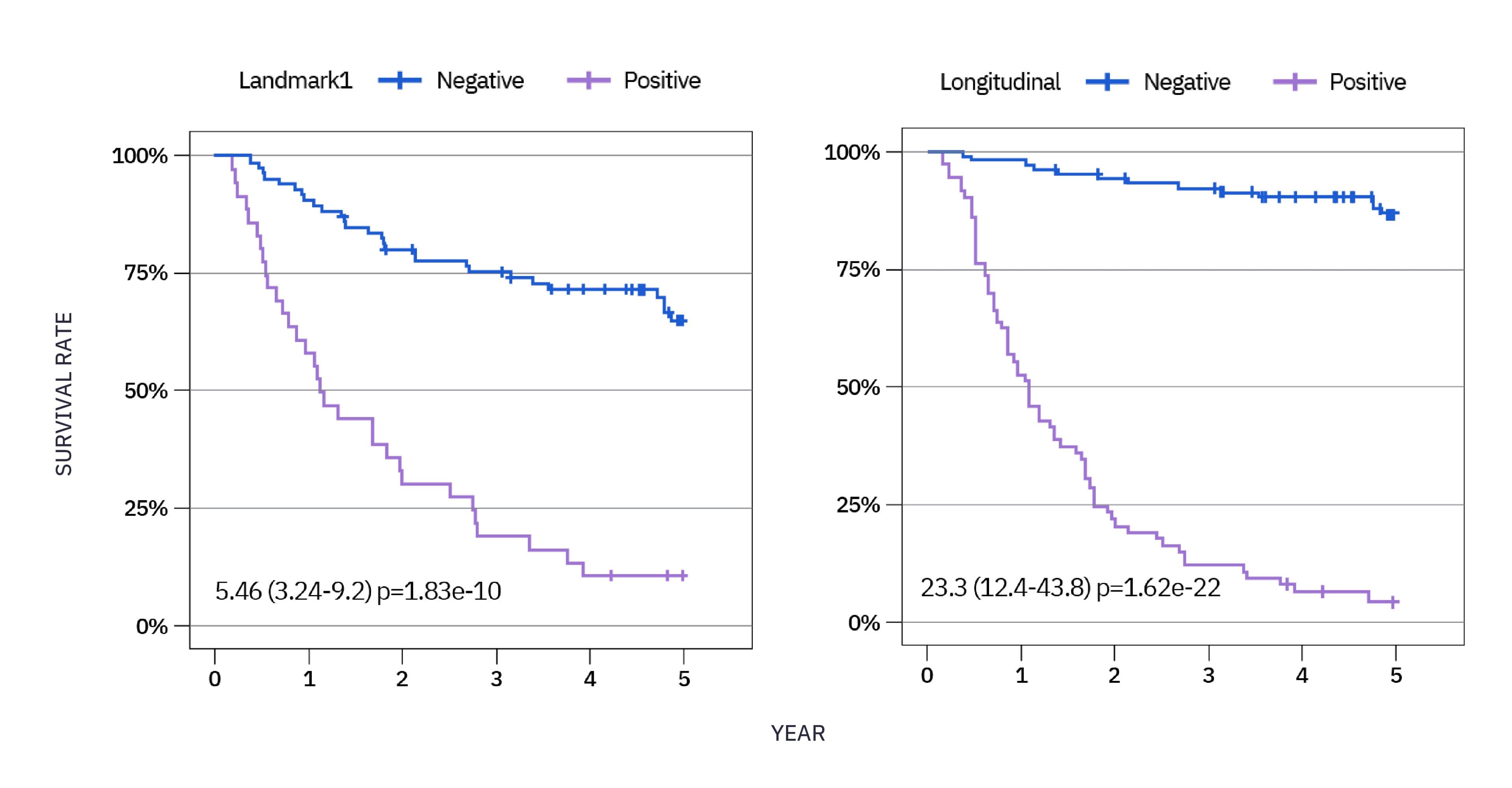
xM (NeXT Personal® Dx) CLINICAL PERFORMANCE IN NSCLC 3
As observed in a study in early stage NSCLC over a 5 year period, 85% of patients who recurred had ctDNA detected, with a median lead time of ~6 months ahead of clinical relapse.*
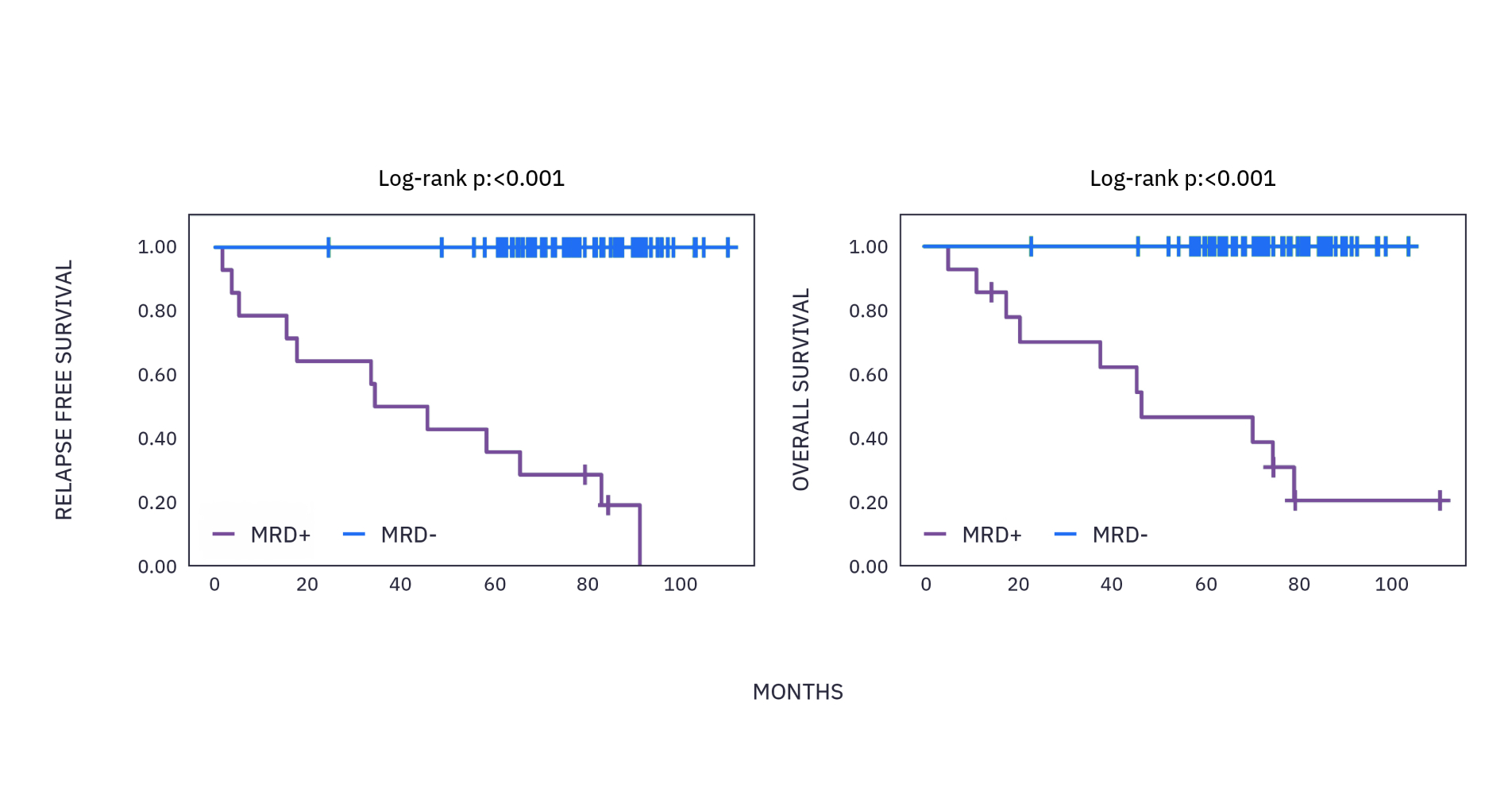
xM (NEXT PERSONAL® DX) CLINICAL PERFORMANCE IN BREAST CANCER 4
As observed in a study in early stage breast cancer over a 6 year period, >99% of patients who recurred had ctDNA detected with a median lead time of ~15 months ahead of clinical relapse.*
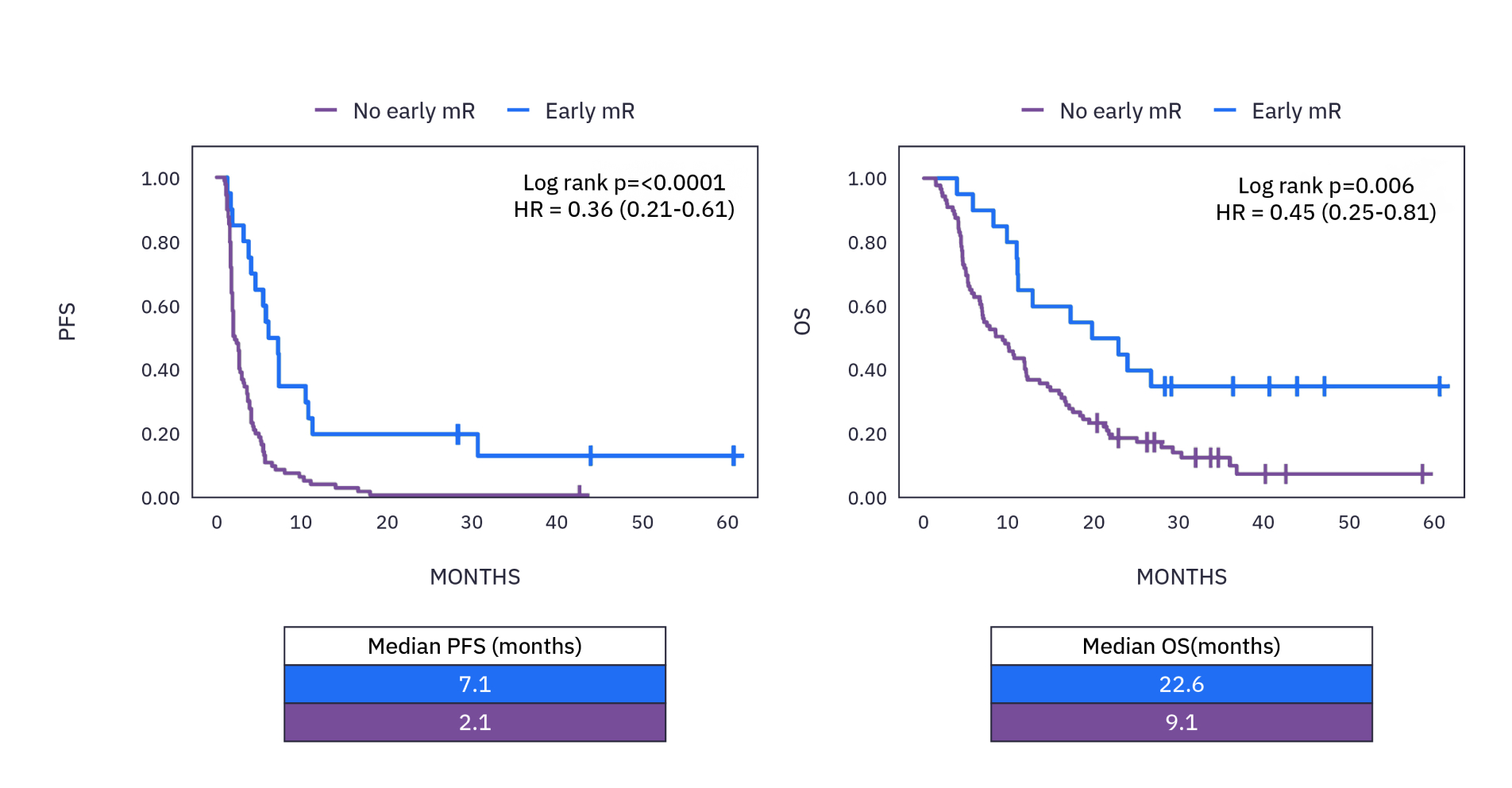
xM (NEXT PERSONAL® DX) CLINICAL PERFORMANCE IN IO MONITORING 5
Patients with metastatic disease in a pan-cancer cohort receiving IO treatment who saw a molecular response (≥30% decrease in ctDNA by cycle 3) had an overall survival that was more than double that of those without a molecular response.
Improving your workflows
Tempus offers a variety of options to customize MRD testing for patients.
ORDERING FLEXIBILITY
Place a single MRD order or a series of longitudinal orders, based on a cadence you determine is medically necessary.
MOBILE PHLEBOTOMY
We want to make blood draw services as convenient as possible for your patients who are unable to come into typical clinical/hospital settings.
To schedule an appointment, please call 800.739.4137 or utilize the Mobile Phlebotomy Request Form.

TEMPUS HUB
Order and view results through our AI-enabled online platform. Paper requisition and EHR ordering are also available.
Access Tempus Hub
Financial Assistance
We help provide access to our tests for all patients in financial need.
Approval of the financial assistance application is based on your household income and takes into account all life circumstances. Once a financial assistance application is submitted either online or over the phone, you will receive a decision at the time of submission.
-
Step 1
Apply for financial assistance online at access.tempus.com.
-
Step 2
If approved, you will know immediately about the maximum out-of-pocket cost of your testing.
-
Step 3
Please contact billing@tempus.com if you are concerned about out-of-pocket costs and would like to discuss your options.
All U.S.-based patients are eligible to apply for financial assistance regardless of insurance status. For uninsured and international patients, we offer a self-pay option. If you have any questions, please email patients@tempus.com.
- Northcott J, Bartha G, Harris J, et al. Analytical validation of NeXT Personal ®, an ultra-sensitive personalized circulating tumor DNA assay. Oncotarget. 2024;15:200-218.
- Nakamura Y, Kaneva K, Lo C, et al. A tumor-naive ctDNA assay detects minimal residual disease in resected stage II or III colorectal cancer and predicts recurrence: subset analysis from the GALAXY study in CIRCULATE-Japan. Clin Cancer Res. 2025;31(2):328-338.
- Black JRM, et al. An ultra-sensitive and specific ctDNA assay provides novel pre-operative disease stratification in early stage lung cancer. Ann Oncol. 2023;34(Suppl):S1294-S1294.
- Garcia-Murillas I, et al. Ultra-sensitive ctDNA mutation tracking to identify molecular residual disease and predict relapse in early breast cancer patients. Presented at: ASCO Annual Meeting; June 2, 2024; Chicago, IL.
- Based on a study involving a cohort of randomly selected metastatic patients from 18 different cancer types, who received 1-3 successive lines of ICIs in the context of phase 1 clinical trials. Toledo R, et al. Prognostic and predictive value of ultrasensitive ctDNA monitoring in a metastatic pan-cancer cohort treated with immune checkpoint inhibitors in the context of phase 1 clinical trials. Presented at: ASCO Annual Meeting; June 4, 2024; Chicago, IL.
*Data listed is longitudinal data. Longitudinal data encompasses landmark data and refers to a minimum of one MRD test performed post-surgery.
This is data-driven precision medicine
This is the future of healthcare.