-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
Tempus xM Monitor (RUO)
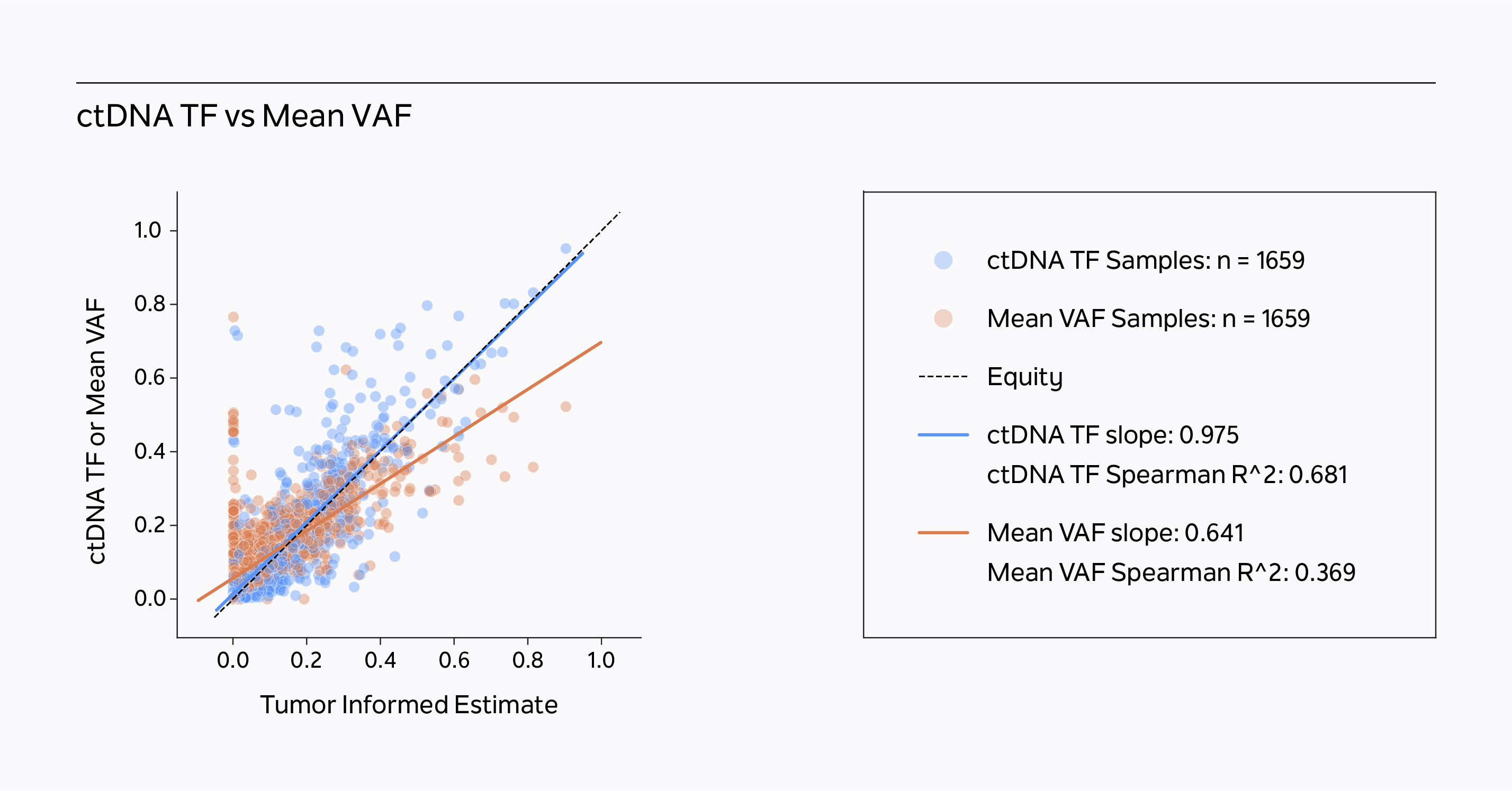
A novel solution for estimating ctDNA Tumor Fraction
xM Monitor measures quantitative molecular changes by utilizing diverse genomic events, dynamically weighting somatic VAFs and copy number variants (CNV), while using germline information to inform these estimates. The ctDNA tumor fraction algorithm utilizes multiple single input models and weights their importance based on failure modes observed in Tempus’ multimodal database. As a result, xM Monitor outperforms mean VAF models.1
Contact us
ADVANCED ALGORITHMIC MODEL
xM Monitor outperforms mean VAF models.
Tempus’ ctDNA tumor fraction algorithmic model correlates with a tumor informed estimate of circulating tumor fraction better than mean VAF in a historical clinical tumor sample dataset.1
Early response
Enables responder identification at an earlier time point compared to standard imaging — potentially as early as post-cycle 1 of immunotherapy.
Monitor resistance mechanisms
Provides comprehensive coverage for emergence of new clonal variants or gene mutations that may be primary drivers of anti-cancer drug resistance.2
Improved estimate of ctDNA tumor fraction
xM Monitor’s ctDNA tumor fraction algorithmic model more accurately calculates ctDNA tumor fraction than mean VAF models, outperforming single-input models.1
Our Science
Association of a novel ctDNA tumor fraction biomarker of treatment response monitoring and clinical outcomes in a real-world, diverse pan-cancer cohort treated with immunotherapyA ≥50% reduction in xM Monitor ctDNA tumor fraction correlates strongly with real-world overall survival (rwOS) in patients receiving either immune checkpoint inhibitor (ICI) monotherapy (Figure a, n=28) or ICI +/- chemotherapy (Figure b, n=86).
Learn more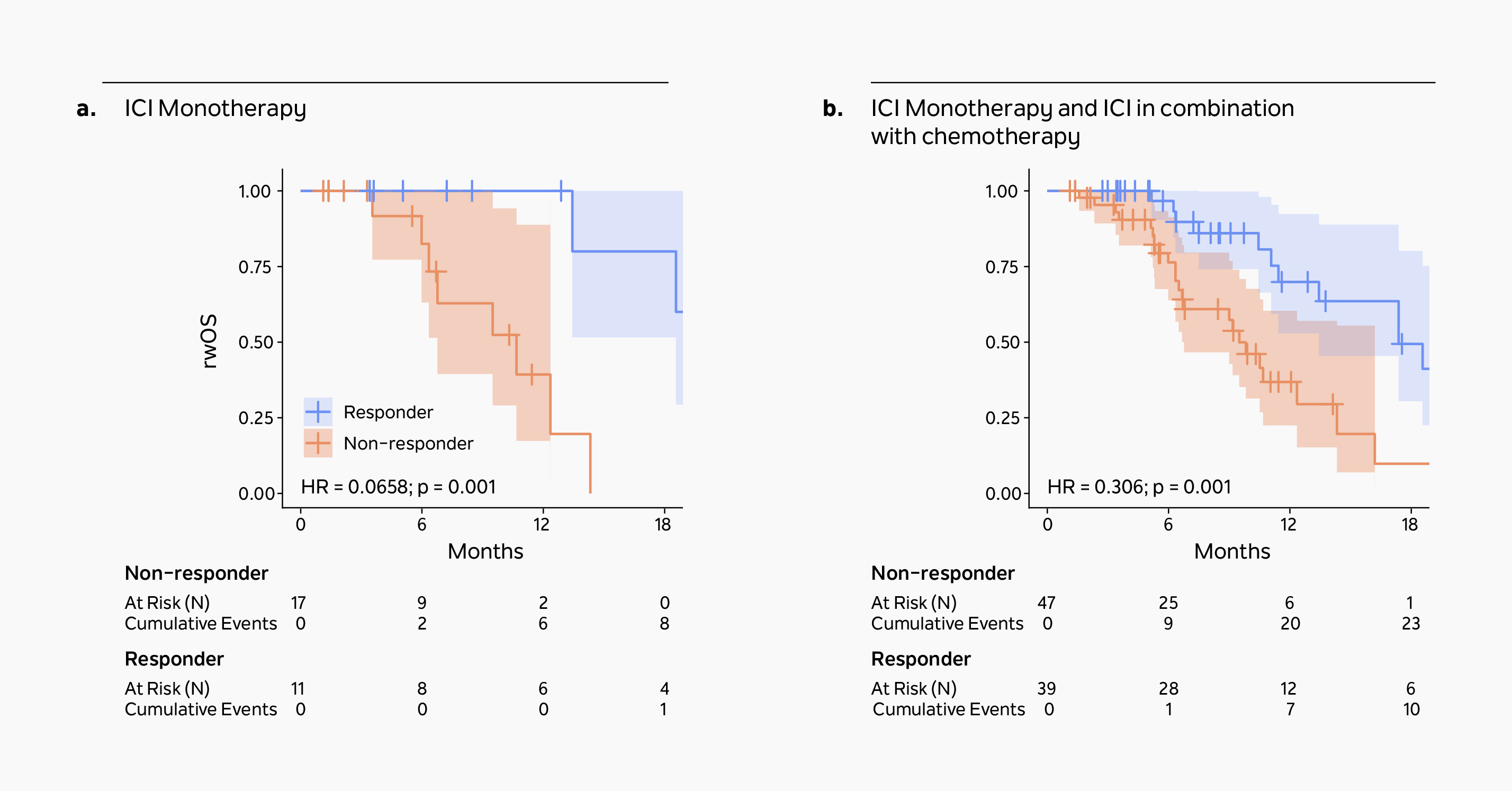
Our suite of ctDNA assays
Tempus xM MRD
MRD Detection
A tumor-naïve assay that detects ctDNA in patients following curative intent treatment to identify patients at high risk of recurrence that may benefit from intensive and/or novel therapy. Currently available for research use only in colorectal cancer.
Tempus xM Monitor
ctDNA Tumor Fraction Calculation & Monitoring
A ctDNA assay which measures changes in ctDNA tumor fraction to determine early response to immunotherapy for patients with advanced cancers. Currently available for research use only.
Tempus xF+
523 Gene Liquid Assay
A ctDNA seq panel that interrogates 523 genes focused on oncogenic and resistance mutations in cell-free DNA (cfDNA) to help identify therapy opportunities. Identifies SNV, insertions / deletions, CNVs, gene rearrangements from select genes, as well as MSI and bTMB.
Tempus xF
105 Gene Liquid Assay
A ctDNA seq panel that detects oncogenic drivers and resistance mutations, assesses for MSI, and identifies SNVs, INDELs, and CNVs.
- xF ctDNA tumor fraction has improved slope and correlation with the Tumor Informed Estimate than the Mean VAF in a historical, pan-cancer tumor sample population (n = 1659 patients). The Tumor Informed Estimate is calculated from xF VAF data informed by corresponding FFPE tumor/normal somatic variant data. The Mean VAF is calculated from xF clinically reportable somatic variant data. Guittar J, Akash M , Lo C , Benshachar R, Nimeiri H et al., 2023.
- Zhong, L., Li, Y., Xiong, L. et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Sig Transduct Target Ther 6, 201 (2021).
This is data-driven precision medicine
This is the future of healthcare.