-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
ENROLL NOW
Tempus’ patient-derived organoid screens
Evaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
10/10/2024
OneOncology
Tempus is collaborating with OneOncology, the fastest-growing national platform for independent oncology practices, to broaden patient access to cutting-edge clinical trials. As part of the collaboration, Tempus is combining the power of its TIME Trial® Program with OneOncology’s robust research infrastructure and large national network of oncology practice partners to bring more biomarker-driven trials to patients in the community setting.
Tempus launched TIME in 2019 in an effort to bring clinical trials to patients in communities across the U.S., building a standardized operational framework and a just-in-time network that brings trials to patients in an average of approximately 10 days. Since then, TIME has seamlessly connected patients, their providers, and trial sponsors to enroll and activate trials for patients closer to home in a fraction of the time it has historically taken. Tempus has already enabled over one thousand patients in the community to be consented into TIME trials. Tempus and OneOncology aim to accelerate that momentum to ensure that the right patients are matched to the right trial at the right time.
OneOncology, with its nationwide network of leading oncology practice partners, is uniquely positioned to bring a growing range of clinical trials to patients who receive cancer care in their own communities. This partnership will enable more patients to participate in trials that might otherwise be unavailable to them. Expanded access to clinical trials in oncology practices also helps to accelerate the development of new therapies and improve patient outcomes.
This collaboration underscores Tempus and OneOncology’s mutual commitment to advancing cancer care and ensuring that more patients have access to the latest therapies. By combining Tempus’ expertise in rapid trial activation with OneOncology’s robust network of community oncology practices, the two organizations are paving the way for a more efficient and patient-centric approach to clinical trials.
-
12/04/2025
Pioneering decentralized oncology trials: Success at the nation’s largest community practices
Hear directly from industry leaders and pioneering site partners as they share insights on expanding their research footprint and improving financial sustainability for their institutions.
Watch replay -
11/11/2025
A new era of biopharma R&D: The TechBio revolution—realities and the next frontier
Join Tempus and Recursion leaders to explore their strategic TechBio partnership. Learn how they use AI and supercomputing with petabytes of data to accelerate drug discovery and development. See the impact on biopharma R&D's future.
Watch replay -
11/14/2025
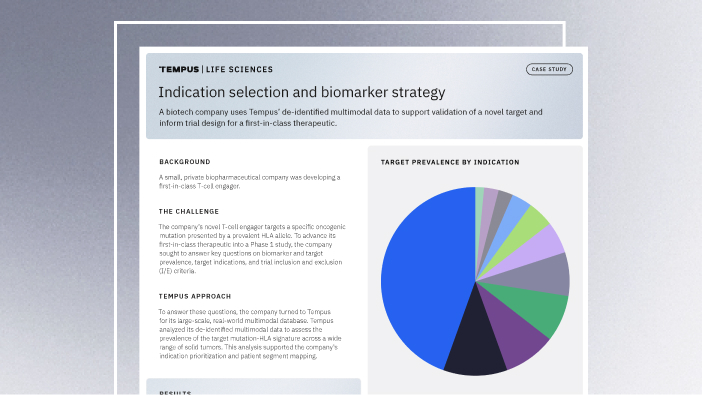
Validating a novel target and informing trial design for a first-in-class therapeutic
Discover how a biopharma company used Tempus’ de-identified multimodal data to support validation of a novel target and inform trial design for a first-in-class therapeutic.
Read more