-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
ENROLL NOW
Tempus’ patient-derived organoid screens
Evaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
ONCOLOGY /// ALGORITHMIC TESTS
The Tempus algorithmic test platform leverages our molecular and clinical database, CAP/CLIA lab, and clinician network to develop tests that help inform the treatment of cancer patients.
Laboratory Developed Tests
IPS TEST
The Tempus Immune Profile Score (IPS) is a multimodal biomarker that can be used as a prognostic indicator for adult patients with metastatic and/or stage IV pan-solid tumor disease who are already considered candidates for immune checkpoint inhibitor (ICI) based therapy.
IPS uses DNA and RNA sequencing data from the Tempus xT CDx and xR tests to classify patients as IPS-High or IPS-Low. Patients classified as IPS-High were found to have a higher real world overall survival (rwOS) compared to those with an IPS-Low result when treated with ICI-based regimens. IPS may provide enhanced insights complementary to standard biomarker testing used today.
Learn more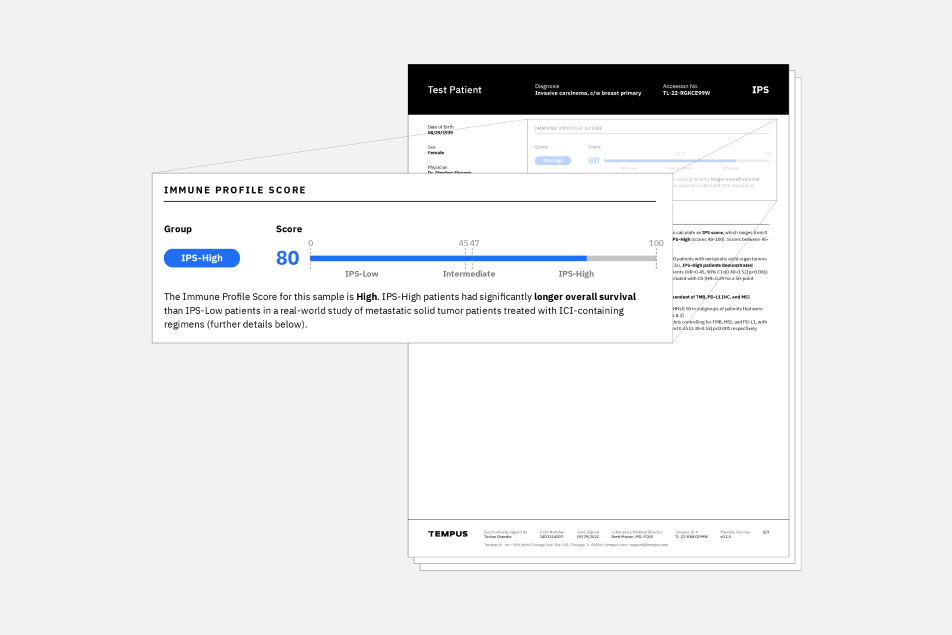
Tempus IPS Advantages
No additional tissue required
IPS may be ordered as an add-on with xT CDx & xR combination OR xT & xR combination.
Both DNA and xR RNA seq are required for processing
Clinical Applications
Validated performance of IPS demonstrated that it is prognostic in the following subgroups: PD-L1 (positive/negative), TMB (high/low), and microsatellite instability status (MSS/MSI-high).
AI-DRIVEN HRD TEST
Tempus HRD is a laboratory developed test to predict the probability of a patient’s cancer having a phenotype characterized by the inability to repair DNA breaks via the homologous recombination repair (HRR) pathway, known as homologous recombination deficiency (HRD). It is available as an additional test for patients who are tested with Tempus xT CDx, xT, or xR.
For ovarian and breast cancer, where DNA based methods of HRD detection are common or under investigation, Tempus HRD provides a result based on DNA genome-wide loss of heterozygosity (GWLOH) or evidence of biallelic BRCA1 or BRCA2 loss from the xT CDx test. For patients with other cancers, Tempus HRD provides an HRD score based on whole transcriptome RNA expression using data from the xR test.
Learn more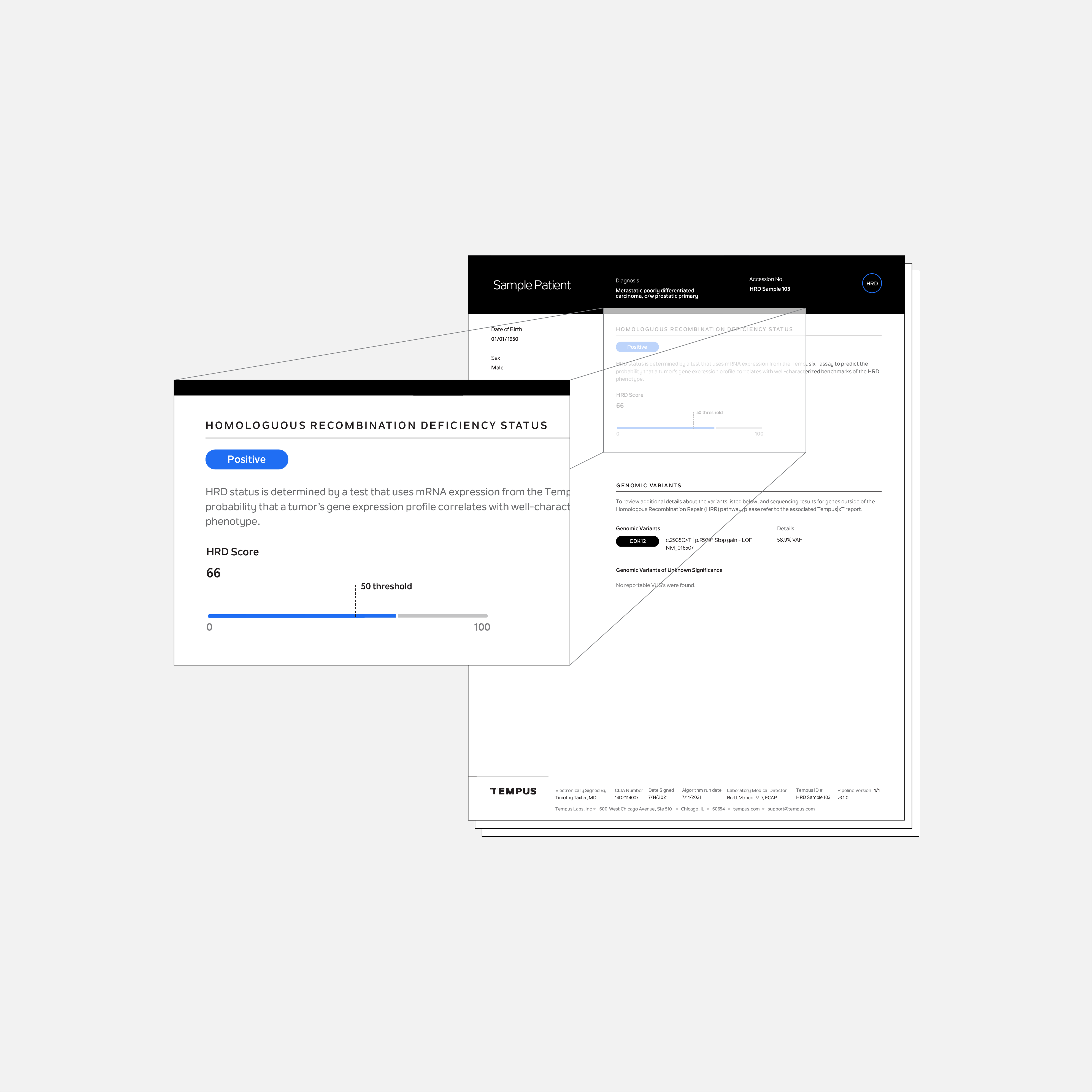
Tempus HRD Advantages
No additional tissue required
HRD may be ordered as an add-on with xT CDx & xR combination OR xT & xR combination OR xR RNA seq.
The matched normal sample is required for breast and ovarian cancer.
xR RNA seq is required for processing in all other cancers.
A more complete patient view in one test
Gain additional insight into a patient’s tumor molecular phenotype on top of the genomic profiling results assessed by xT CDx, xT, or xR.
AI-DRIVEN TO TEST
The Tempus Tumor Origin (TO) test uses tumor RNA expression results to predict the patient’s most likely cancer type(s) from 68 possible cancer types. The Tempus TO test was developed using a large internal database of clinical and annotated molecular tumor data.
The intended use of the TO test is for cancers of unknown primary and cases for which the available diagnostic information, such as imaging and immunohistochemistry results, do not provide a definitive diagnosis. When paired with xR, the TO results may provide insight into the patient’s diagnosis and tumor site of origin that may be used to inform patient care and clinical trial eligibility.
Learn more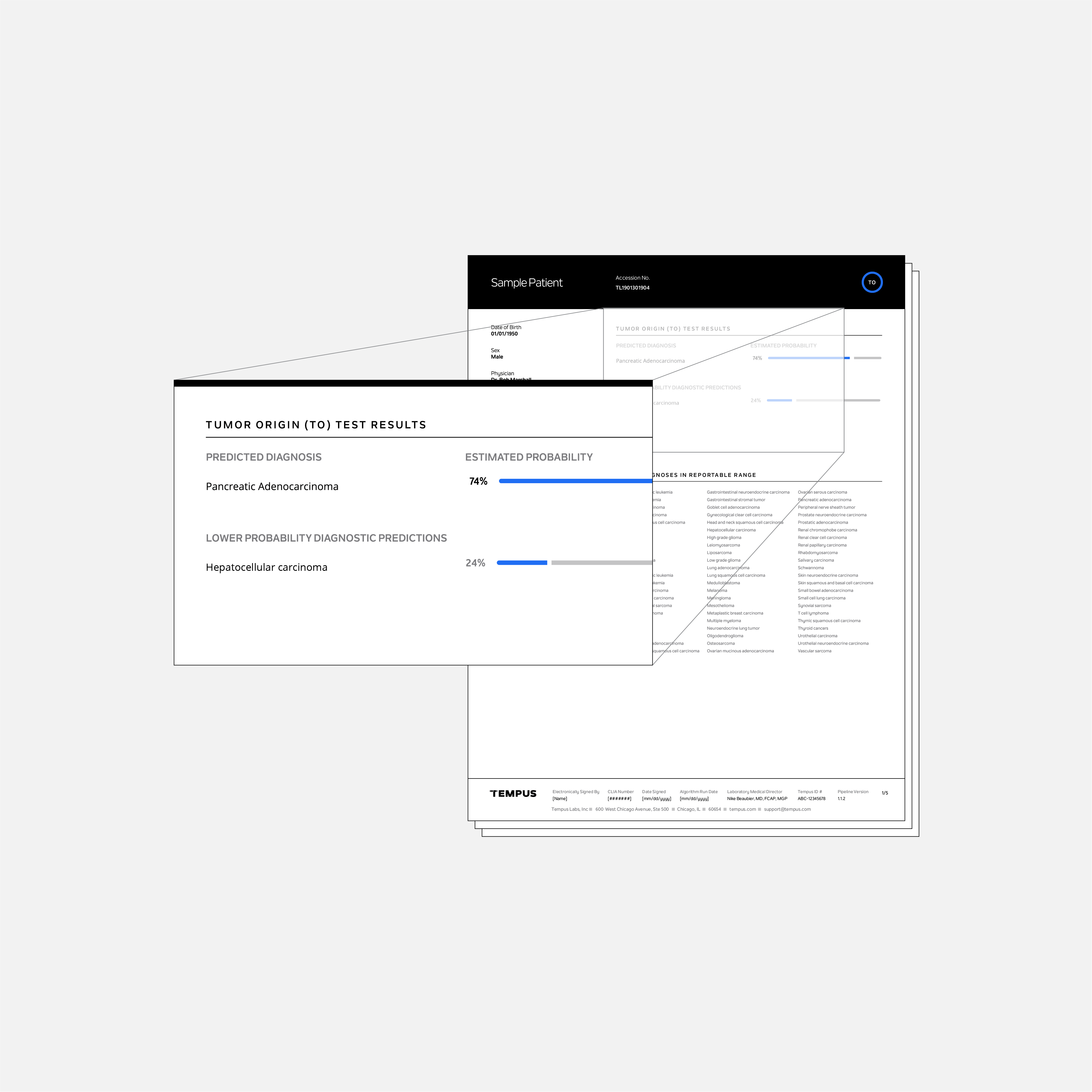
Tempus TO Advantages
No additional tissue required
TO can be ordered as an add-on with xT CDx & xR combination OR xT & xR Combination OR xR RNA seq.
xR RNA seq is required for processing.
Coverage of many cancer types
The test characterizes 68 possible cancer types and subtypes to provide precise information for guiding treatment decisions.
DPYD TEST
The DPYD gene encodes the enzyme dihydropyrimidine dehydrogenase (DPD), a key protein involved in 5-FU/Capecitabine metabolism.
The Tempus DPYD test is designed to help physicians to better identify patients who may be at elevated risk for toxicity and serious adverse effects from 5-FU and capecitabine. It is applicable in situations where 5-FU and/or capecitabine treatments are considered, especially colorectal, breast, pancreatic, and other GI cancers.
Learn more
Tempus DPYD Advantages
No additional tissue required
DPYD can be ordered as an add-on with xT CDx & xR combination.
The matched normal sample is required for processing.
Expanded toxicity risk assessment
DPYD analysis covers five SNVs in DPYD genes, providing a more complete patient profile.
UGT1A1 TEST
The Tempus UGT1A1 test identifies patients at elevated risk for toxicity from treatment with irinotecan, sacituzumab govitecan, and/or belinostat.
The Tempus UGT1A1 test identifies certain genetic polymorphisms in the UGT1A1 gene. These polymorphisms are associated with an elevated risk of developing toxicity to irinotecan, sacituzumab govitecan, and/or belinostat based on drug labeling. A patient who has one of the following genotypes will receive a positive test result: *28/*28, *6/*6, and *6/*28.
Learn more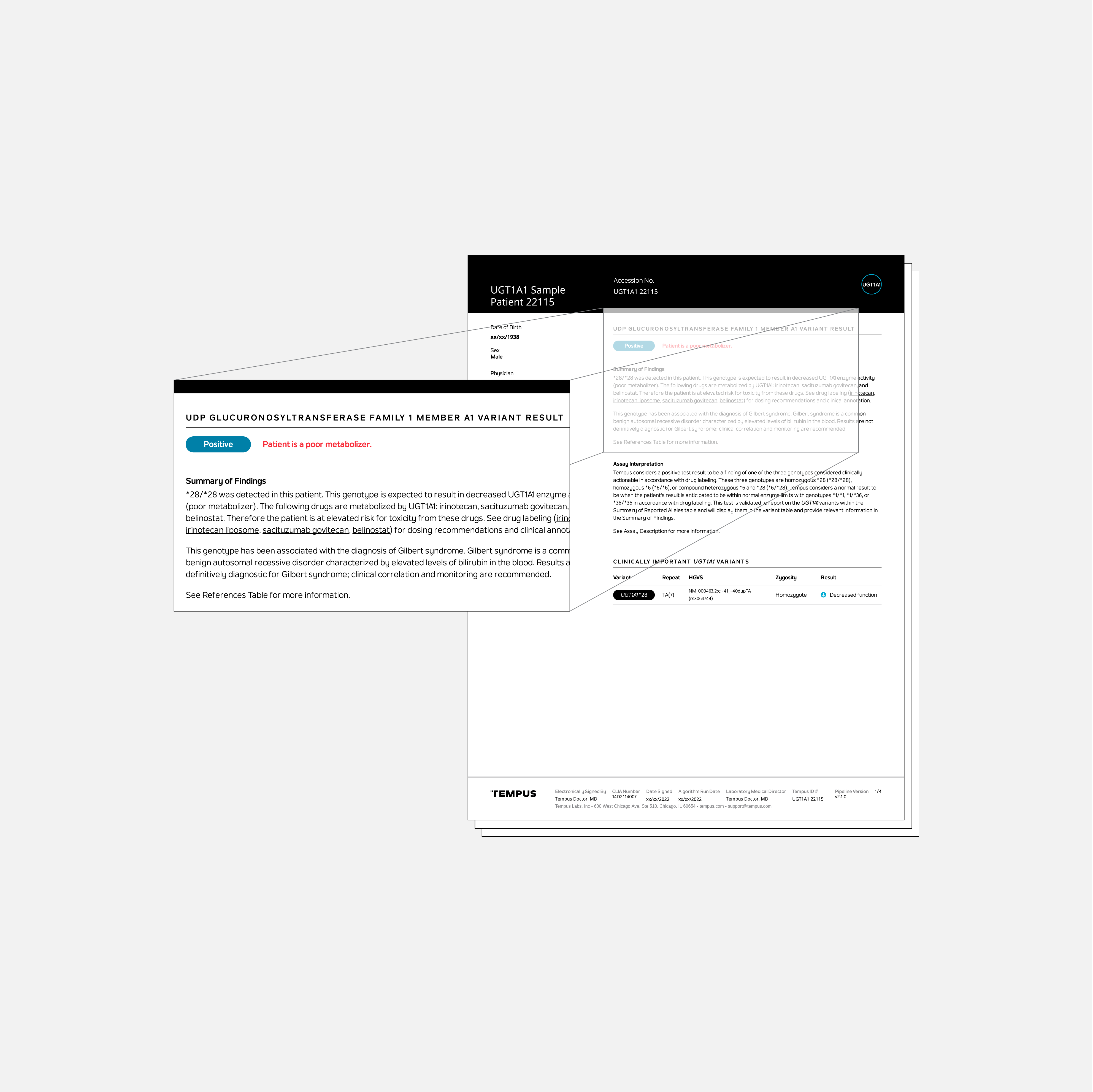
Tempus UGT1A1 Advantages
No additional tissue required
UGT1A1 can be ordered as an add-on with xT CDx & xR combination.
The matched normal sample is required for processing.
Expanded toxicity risk assessment
The Tempus UGT1A1 test identifies 5 variants from 3 locations on the UGT1A1 gene, for a complete patient profile.
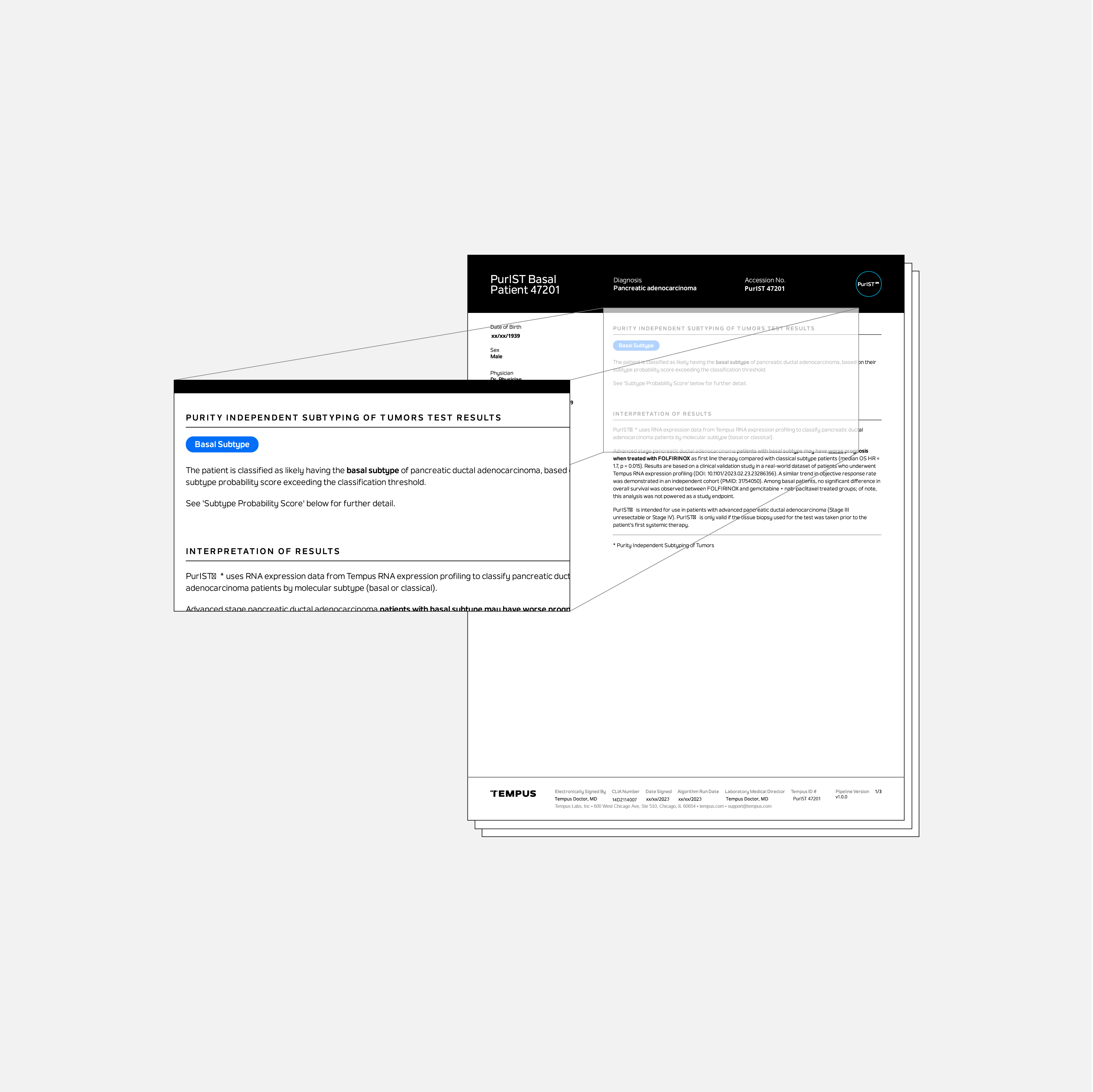
PurIST®
Tempus’ PurIST® test is a laboratory developed test that uses an RNA-based algorithm to classify pancreatic ductal adenocarcinomas (PDAC) into one of two subtypes (basal-like or classical).
Published literature using the PurIST® algorithm with non-Tempus RNA sequencing data indicates that patients with the basal subtype have a worse prognosis and are less likely to benefit from FOLFIRINOX therapy than classical patients.1
Tempus independently validated use of the PurIST® algorithm with Tempus RNA sequencing data which demonstrated subtype stratification with distinct survival outcomes and treatment benefit, supporting the integration of PurIST® into routine clinical care for patients with advanced PDAC to inform first-line chemotherapy selection.2
Learn more1. Rashid NU, et al. Purity independent subtyping of tumors (PurIST), a clinically robust, single-sample classifier for tumor subtyping in pancreatic cancer. Clin Cancer Res. 2020;26(1):82-92. doi:10.1158/1078-0432.CCR-19-1467.
2. Wenric S, Sangli C, Guittar J, et al. Real-World Validation of the Purity Independent Subtyping of Tumors Classifier for Informing Therapy Selection in Pancreatic Ductal Adenocarcinoma. JCO Precis Oncol. 2025;9:e2500197. doi:10.1200/PO-25-00197
Tempus PurIST Advantages
No additional tissue required
PurIST can be ordered as an add-on with xT CDx & xR combination OR xT & xR combination OR xR RNA seq.
xR RNA seq is required for processing.
A more complete patient view in one test
Gain additional insight into a patient’s tumor molecular phenotype on top of the genomic profiling results assessed by xR.