-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
ENROLL NOW
Tempus’ patient-derived organoid screens
Evaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
11/05/2024
Clinical Validation of a Novel Multi-Omic Algorithm for Stratifying Outcomes in a Real-World Cohort of Metastatic Solid Cancer Patients Treated With Immune Checkpoint Inhibitors
SITC 2024
PRESENTATION
Authors
Alia Zander, PhD , Rossin Erbe, PhD , Yan Liu, PhD , Ailin Jin , Seung W Hyun, PhD , Sayantoni Mukhopadhyay , Ben Terdich , Mario G Rosasco,PhD , Nirali Patel, MD , Brett Mahon, MD , Kate Sasser, PhD , Michelle Ting-Lin, MD , Halla Nimeiri, MD , Justin Guinney, PhD , Kyle Beauchamp,PhD , Chithra Sangli , Michelle M Stein, PhD , Timothy Taxter, MD, Timothy Chan, MD, PhD , Sandip P Patel, MD and Ezra E. W. Cohen, MD
Background – Despite advances in immune checkpoint inhibitor (ICI) biomarker molecular
testing, there remains an unmet clinical need for more sensitive and generalizable biomarkers to better predict patient outcomes to ICI. This has been challenging due to the limited availability of multi-omic testing and validation cohorts. An integrated DNA/RNA ICI biomarker can address this critical unmet need.
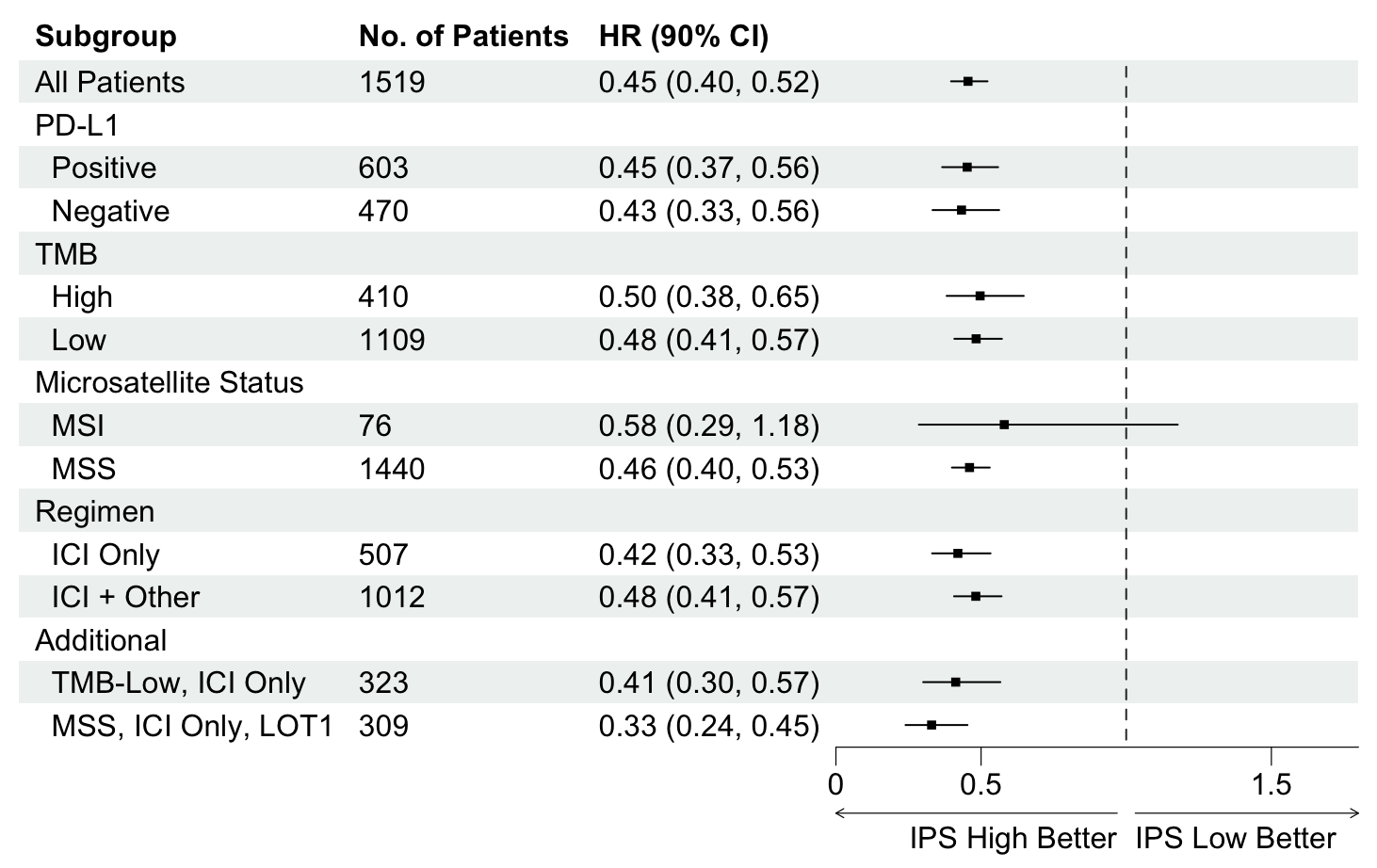
Methods – A de-identified pan-cancer cohort from the Tempus multimodal real-world database was used for the development and validation of the Immune Profile Score (IPS) algorithm leveraging Tempus xT (648 gene DNA panel) and xR (RNAseq). The cohort (n=1707 training [T]; n=1519 validation [V]) consisted of advanced stage cancer patients treated with any ICI containing regimen as the first or second line of therapy (LOT). The IPS model was developed utilizing a machine learning framework that includes tumor mutational burden (TMB) and 8 RNA-based biomarkers as features. Cox models were fit to demonstrate prognostic utility. Predictive utility of IPS was evaluated using a Cox model for recurrent events.
Results – IPS-high patients demonstrated significantly longer overall survival (OS) compared to IPS-low patients (HR=0.45, 90% confidence interval = [0.40-0.52]). IPS was consistently prognostic in the PD-L1 (positive/negative), TMB (high/low), microsatellite status (MSS/MSI), and regimen (ICI only/ICI + other) subgroups (Figure 1). In the subgroup of TMB-low patients who received ICI only therapy (n= 323), IPS-high patients had longer survival than IPS-low patients (HR=0.41 [0.30-0.57]). Additionally in the subgroup of MSS patients who received ICI only therapy in LOT1, IPS-high patients had longer survival then IPS-low patients (HR=0.33 [0.24-0.45]). Additionally, IPS remained significant in multivariable models controlling for TMB, MSI, and PD-L1, with IPS HRs of 0.49 [0.42-0.56], 0.47 [0.41-0.53], and 0.45 [0.38-0.53] respectively. In an exploratory IPS predictive utility analysis of the subset of T+V patients (n=345) receiving chemotherapy (CT) in LOT1 and ICI in LOT2, there was no significant effect of IPS for time to next treatment on CT in LOT1 (HR=1.06 [0.85-1.33]). However, there was a significant effect of IPS for OS on ICI in LOT2 (HR=0.63 [0.46-0.86]). A test of interaction was statistically significant (p<0.01).
Conclusions – Our results demonstrate that IPS is a generalizable multi-omic biomarker that
can be widely utilized clinically as a prognosticator of ICI based regimens. Importantly, IPS-high may identify relevant patient subgroups (TMB-L, MSS, PD-L1 negative) who benefit from ICI beyond what is predicted by existing biomarkers. Moreover, an exploratory analysis is suggestive of predictive utility. Future prospective predictive utility studies are planned.
Learn more about how the Immune Profile Score (IPS) test can support your needs
-
Advance your research and development with IPS
-
Enhance clinical insights with IPS for your advanced cancer patients