-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
11/05/2024
EVEREST-1: Initial Safety Data From a Seamless Phase 1/2 Study of A2B530, a Logic-Gated Tmod Car T-Cell Therapy, in Patients With Solid Tumors Associated With Cea Expression Also Exhibiting HLA-LOH
SITC 2024
PRESENTATION
Authors
Patrick M Grierson, Salman R Punekar, Antonious Hazim, Theodore Welling, Diane M Simeone, Kedar Kirtane, Kian-Huat Lim, Maria Pia Morelli, Sandip Pravin Patel, Matthew Ulrickson, Tiago Biachi de Castria, Madappa Kundranda, Judy Vong, Jasmine Mitchell, Wendy J Langeberg, Marcela Maus, William S Bretzlaff, Sarah Nikiforow, Armen Mardiros, David G Maloney, Kirstin Liechty, Frederick L Locke, Eric W Ng, J Randolph Hecht, William Y Go and Julian R Molina
Background Chimeric antigen receptor T-cell (CAR T) therapy in solid tumors has been challenging, in part, owing to a lack of tumor-specific targets that discriminate cancer from normal cells resulting in dose-limiting, on-target, off-tumor toxicities.1 2 A2B530 is a logic-gated carcinoembryonic antigen (CEA)-targeting TmodTM CAR T therapy being evaluated in the EVEREST-1 (NCT05736731) first-in-human study. Tmod CAR T therapy addresses challenges of on-target, off-tumor toxicity by combining a CEA-targeted CAR-activating receptor with a blocking receptor recognizing human leukocyte antigen (HLA)-A*02 to discriminate tumor from normal cells (figure 1).3 4
Methods Patients with colorectal, pancreatic, or non-small cell lung cancer are enrolled through BASECAMP-1 (NCT04981119), a prescreening study that identifies patients with tumors harboring HLA-A*02 loss of heterozygosity (LOH). BASECAMP-1 eligible patients undergo leukapheresis and, when clinically appropriate, their banked T cells are manufactured for the EVEREST-1 study. Patients undergo lymphodepletion before A2B530 infusion. The initial cohort of the phase 1 dose escalation to evaluate safety, tolerability, manufacturing feasibility, and biomarkers started at 1 x 108 Tmod positive cells (dose level 1) with the highest at 6 x 108 cells along with higher levels of lymphodepleting chemotherapy (dose level 5). After the recommended phase 2 dose (RP2D) has been established, an additional dose expansion will confirm safety and activity.
Results As of May 1, 2024, 10 patients with unresectable locally advanced or metastatic solid tumors expressing CEA and with HLA-A*02 LOH were treated with A2B530 in dose levels 1–4 (figure 2). Lymphodepleting chemotherapy was well tolerated: neutropenia nadired at days 7–10, with recovery in all patients, and no significant anemia or thrombocytopenia was observed. There were no dose-limiting toxicities, grade >3 serious adverse events, nor related neurotoxicity. One patient in dose level 2 (2 x 108 cells) experienced grade 2 cytokine release syndrome, which resolved with supportive therapy. In dose level 2, a 71-year-old female with metastatic pancreatic cancer received gastric radiation 28 days before A2B530 administration. The patient was admitted to the hospital on post infusion day 24, with grade 3 dyspepsia that was preceded by peak serum IFNg, TNFa, and granzyme B. The event resolved in 3 days with medical management. The patient had a confirmed partial response at day 30 and 90 by central evaluation (per Response Evaluation Criteria in Solid Tumors v1.1 on non-irradiated tumors).
Conclusions Treatment with A2B530 has shown manageable toxicity with minimal on-target, off-tumor adverse events and potential clinical efficacy. Dose escalation continues to determine the RP2D.
Acknowledgements The authors would like to thank the following: Patients and their families and caregivers for participating in the study, the screeners, clinical research coordinators, study nurses, data managers, and apheresis teams at all of the study sites, and contributions from others at A2 Bio. Medical writing support was provided by Bio Connections, LLC, and funded by A2 Bio.
Trial Registration NCT05736731.
References
-
Parkhurst MR, et al. Mol Ther 2011;19(3):620–626.
-
Tabernero JT, et al. J Clin Oncol 2017;35(15):3002.
-
Hamburger AE, et al. Mol Immunol 2020;128:298–310.
-
DiAndreth B, et al. Clin Immunol 2022;241:109030.
Ethics Approval This study was approved by site IRBs.
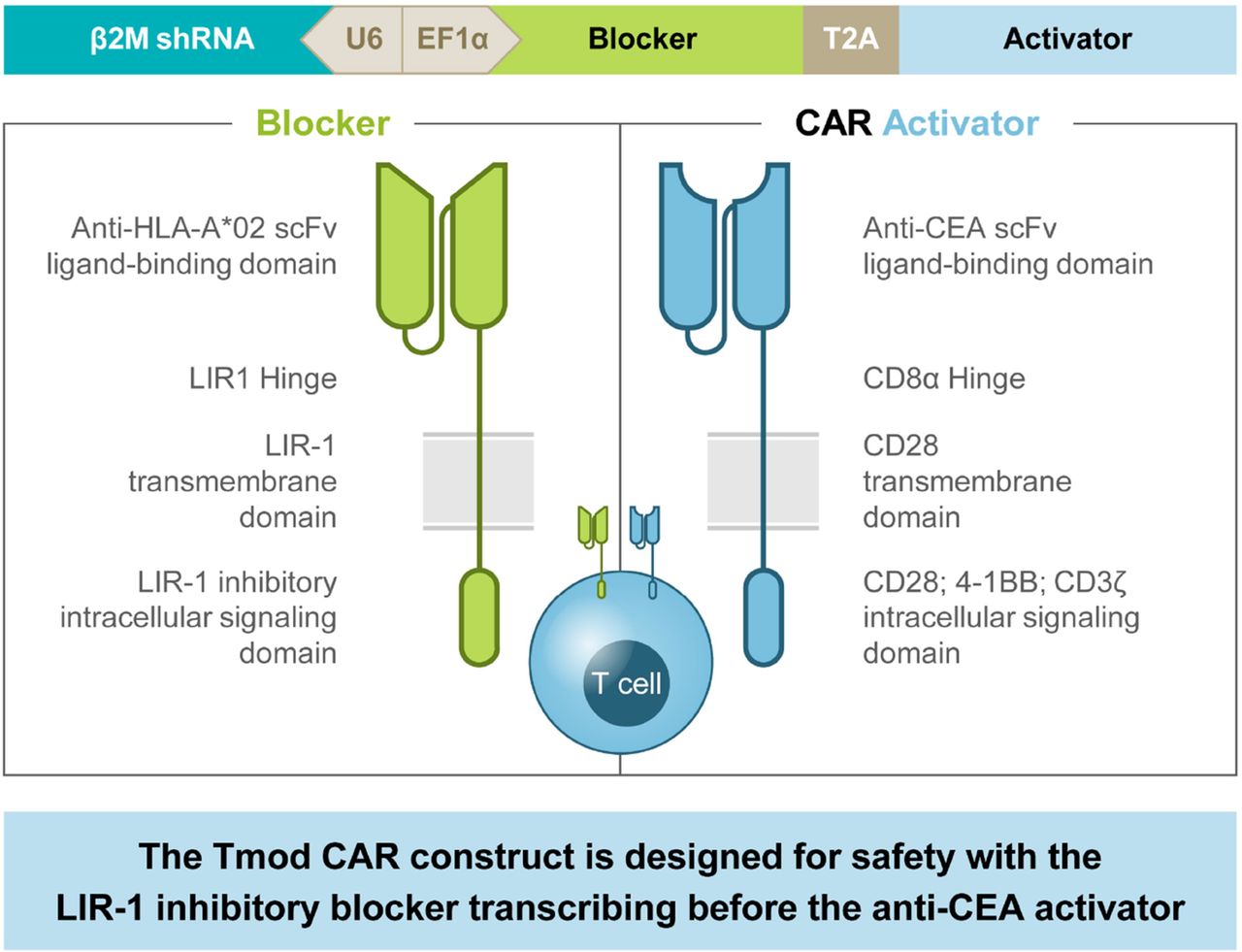
CEA CAR Tmod single vector construct. β2M shRNA, beta–2–microglobulin short–hairpin RNA; CD, cluster of differentiation; EF1α, elongation factor–1 alpha; LIR, leukocyte immunoglobulin–like receptor; scFv, single–chain variable fragment; T2A, thosea asigna virus 2A
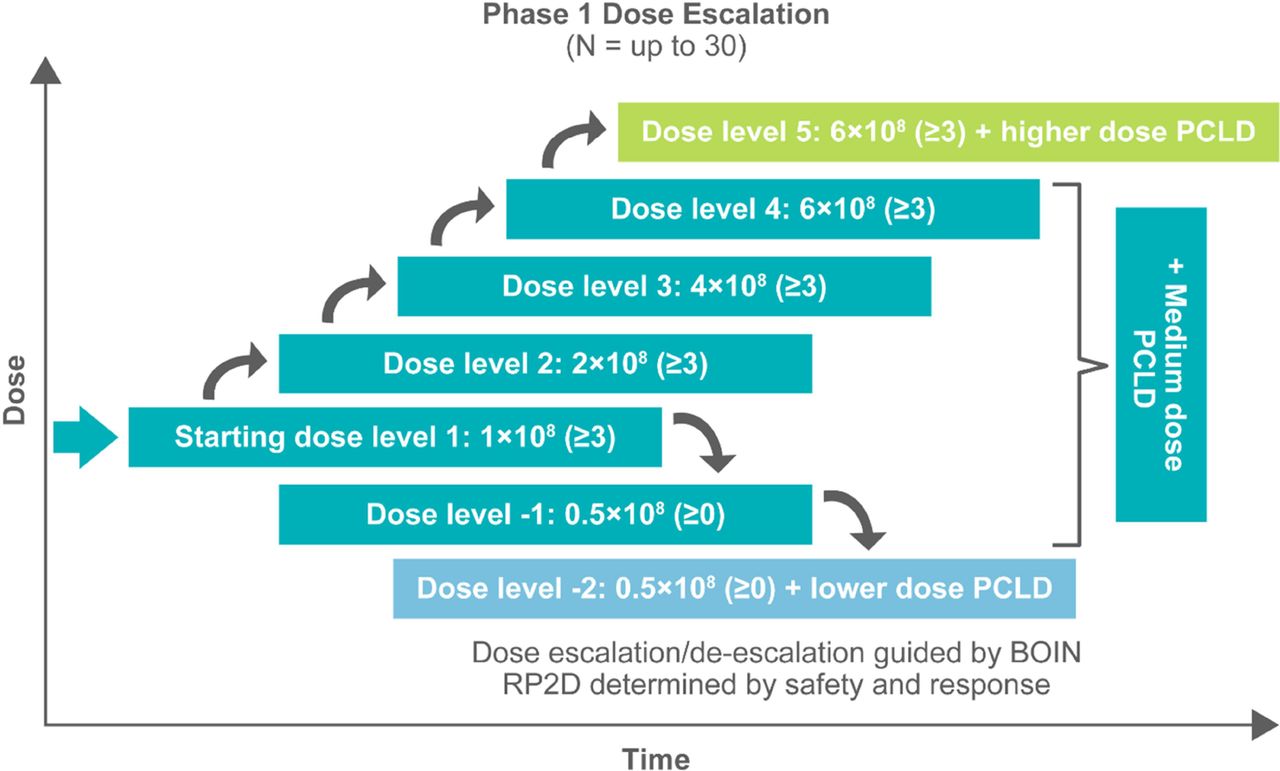
EVEREST-1 study design. BOIN, Bayesian optimal interval design; PCLD, preconditioning lymphodepletion; RP2D, recommended phase 2 dose