-
PROVIDERS
Now Available: xT CDx, FDA-Approved Assay
Discover xT CDx, the FDA-approved assay delivering comprehensive insights through tumor + normal DNA sequencing
- LIFE SCIENCES
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
11/05/2024
EVEREST-2: A Seamless Phase 1/2 Study of A2B694, a Logic-Gated Tmod Car T-Cell Therapy, in Patients With Mesothelin-Expressing Solid Tumors With Human Leukocyte Antigen-A*02 Loss of Heterozygosity
SITC 2024
PRESENTATION
Authors
Julian R Molina, Antonious Hazim, Oliver Dorigo, Salman R Punekar, Monica Avila, Diane M Simeone, Kedar Kirtane, Gottfried E Konecny, Jeffrey P Ward, Matthew Block, Jennifer Specht, Leslie Boyd, Yanyan Lou, Jong Chul Park, Jacqueline D Xuan, Ramez N Eskander, Gayanie Ong, Marco Davila, Wendy J Langeberg, Marcela Maus, Armen Mardiros, Frederick L Locke, Andrea Wise, David G Maloney, John Welch and J Randolph Hecht
Background Chimeric antigen receptor T-cell (CAR T) therapies have demonstrated significant clinical efficacy in hematologic malignancies, but have proven challenging in solid tumors, in part owing to a lack of tumor-specific targets and unmanageable toxicity. Mesothelin (MSLN) expression normally is limited to the mesothelium of major body cavities but can be upregulated in diverse solid tumor types,1 making it a potential target for cancer therapy. MSLN-targeted cell approaches, including CAR T and T-cell receptor fusion therapies, have shown promising clinical activity; however, on-target, off-tumor toxicity including fatal events have occurred.2–4 A2B694 is an autologous logic-gated, MSLN-targeted Tmod CAR T therapy that addresses the challenges of on-target, off-tumor toxicity by combining 2 CARs: an activating and blocking receptor. The activator recognizes MSLN present on the surface of both tumor and normal cells; the blocker prevents CAR T activity when it binds human leukocyte antigen (HLA)-A*02, which is present in normal cells and often lost in tumor cells. Thus, in patients with tumor-associated HLA-A*02 loss of heterozygosity (LOH), the blocker prevents on-target, off-tumor toxicity on normal cells owing to retained HLA-A*02 expression (figure 1).5 Through this unique discriminatory mechanism, A2B694 may provide a therapeutic window to treat patients with MSLN-expressing solid tumors exhibiting HLA-A*02 LOH.
Methods EVEREST-2 (NCT06051695) is a first-in-human, phase 1/2, open-label, nonrandomized study to evaluate the safety and efficacy of A2B694 in adults with recurrent unresectable, locally advanced, or metastatic cancers with MSLN expression, including non-small cell lung cancer, colorectal cancer, pancreatic cancer, ovarian cancer, mesothelioma, or other solid tumors with MSLN expression. Eligible patients must have exhausted options of potentially curative therapy and had a recurrence. Enrollment to EVEREST-2 occurs through the prescreening study BASECAMP-1 (NCT04981119), which identifies patients with tumor-associated HLA-A*02 LOH via next-generation sequencing (Tempus AI, Inc.; figure 2). Eligible patients enroll in the BASECAMP-1 study and undergo leukapheresis. A2B694 is manufactured from cryopreserved T cells when clinically appropriate for patients. The primary objective of phase 1 is to evaluate the safety and tolerability of A2B694 and identify the recommended phase 2 dose (RP2D). The dose-expansion phase will confirm RP2D and collect biomarker data to further characterize A2B694. Phase 2 will assess overall response rate per RECIST v1.1.
Results The first patient was enrolled on EVEREST-2 in April 2024. Dose escalation is ongoing (figure 3).
Acknowledgements The authors would like to thank the following: Patients and their families and caregivers for participating in the study, the screeners, clinical research coordinators, study nurses, data managers, and apheresis teams at all of the study sites, and contributions from others at A2 Bio. Medical writing support was provided by Bio Connections, LLC, and funded by A2 Bio.
Trial Registration NCT06051695.
References
-
The Cancer Genome Atlas (TCGA) Research Network. Accessed June 2021. https://www.cancer.gov/tcga
-
Beatty GL, et al. Gastroenterology. 2018;155(1):29–32.
-
Haas AR, et al. Mol Ther. 2023;31:2309–2325.
-
Hong DS, et al. ESMO 2021; Abstract 9590.
-
Hamburger AE, et al. Mol Immunol. 2020;128:298–310.
Ethics Approval This study was approved by site IRBs.
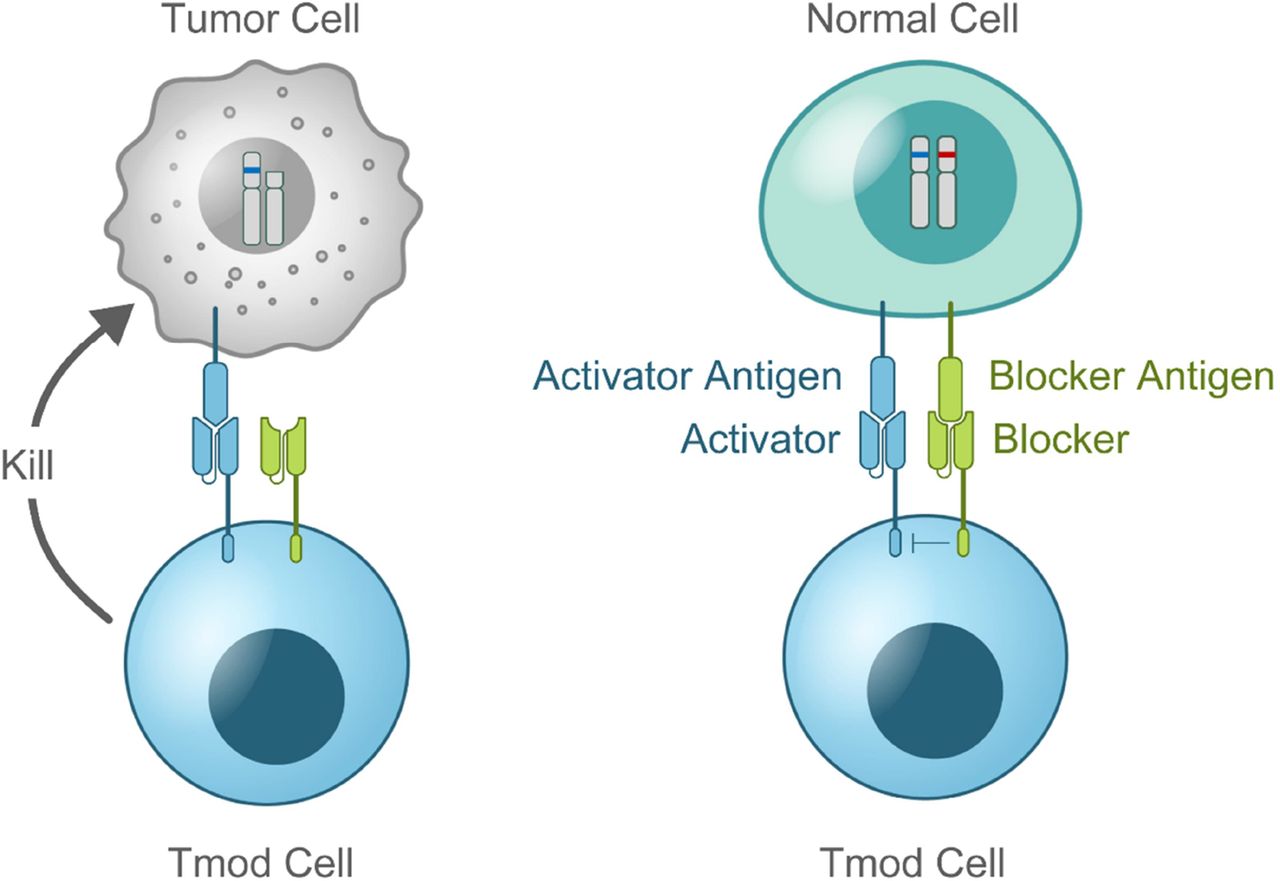
Logic-gated CAR T with the goal to reduce toxicity: MSLN (Activator) and HLA-A*02 (Blocker)5
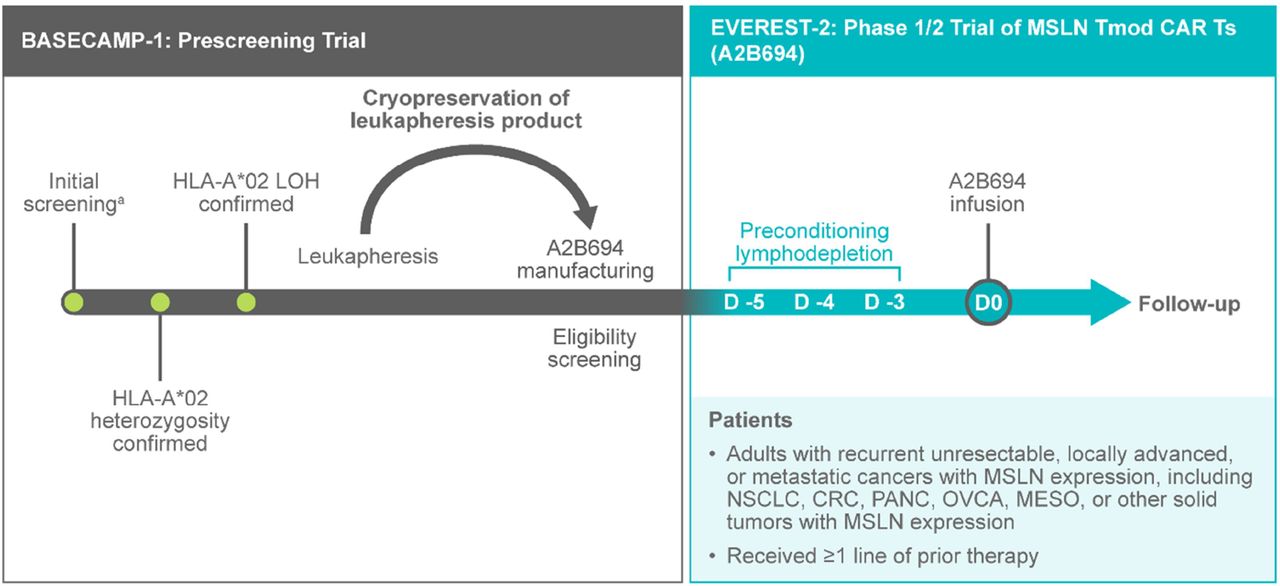
Study schema: BASECAMP-1 to EVEREST-2. a May occur at any point in disease course. CRC, colorectal cancer; D, day; MESO, mesothelioma; NSCLC, non-small cell lung cancer; OVCA, ovarian cancer; PANC, pancreatic cancer; PCLD, preconditioning lymphodepletion
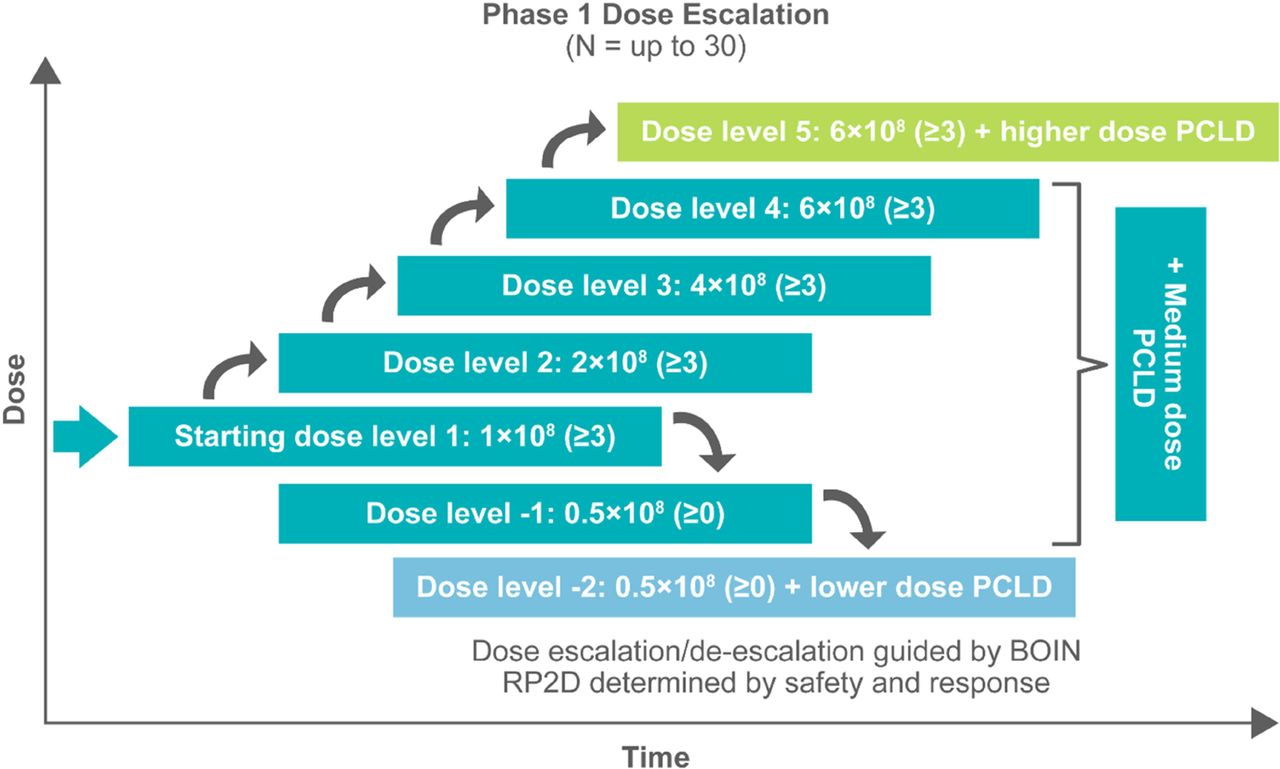 EVEREST-2 phase 1 dose escalation study design
EVEREST-2 phase 1 dose escalation study design