-
PROVIDERS
REGISTER NOW
Navigating new frontiers in breast cancer care pathway intelligence: The role of providers and AI
Tuesday, July 29th
2:00pm PT, 4:00pm CT, 5:00pm ET -
LIFE SCIENCES
REGISTER NOW
Closing Care Gaps with AI: The Next Competitive Edge in Pharma
Monday, July 14
9am PT, 11am CT, 12pm ET -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
RADIOLOGY /// BREAST
AI-enabled solution to screen, detect, and track changes in lesions suspected of breast cancer
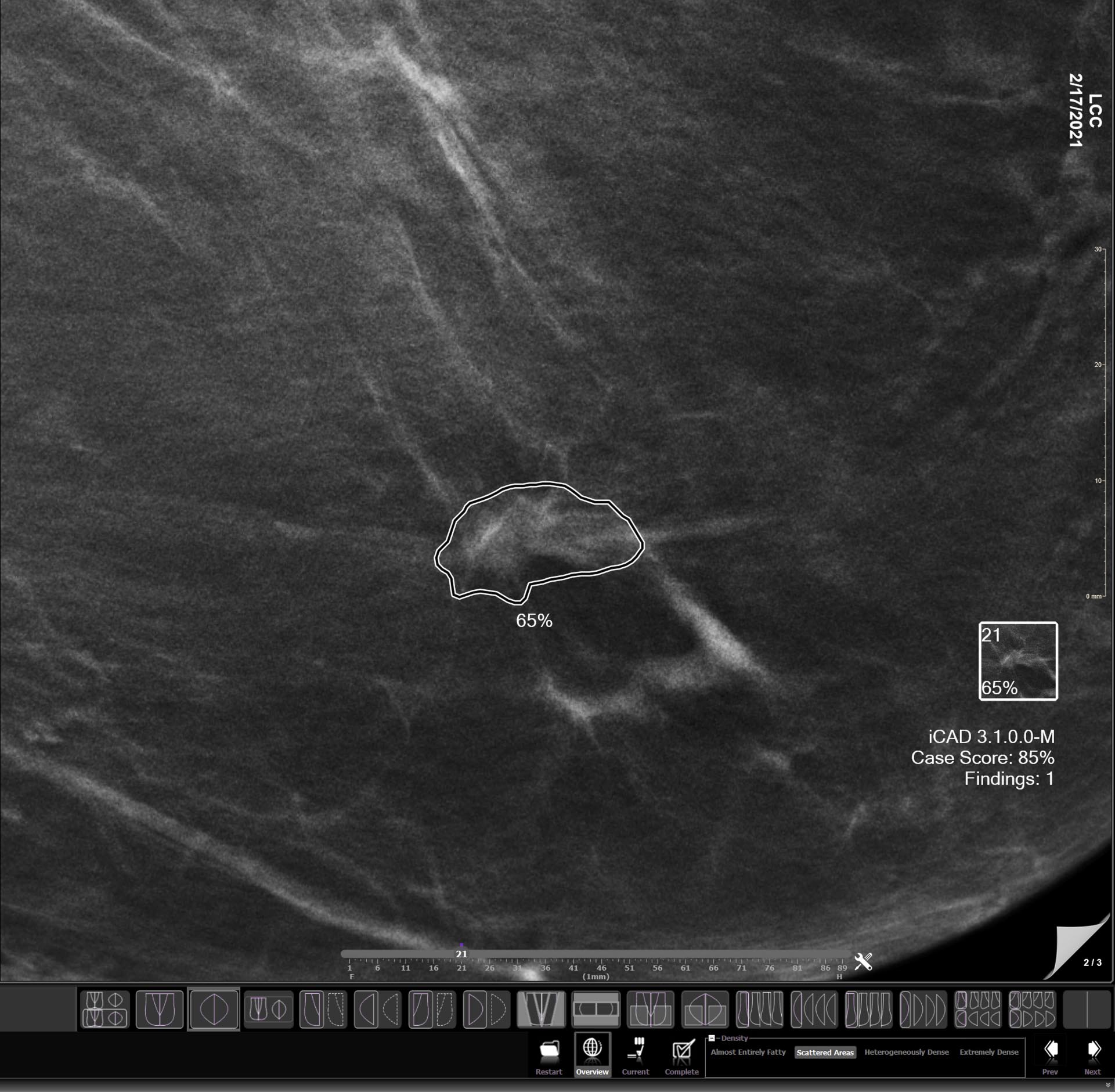
Tempus Pixel provides advanced analysis and automated reporting from routine 2D and 3D mammograms to help improve efficiency and accuracy in detecting and longitudinal tracking of changes in lesions suspected of breast cancer.
Breast lesions identification
Automatically detects lesions suspected of breast cancer in 2D and 3D mammograms.1,2
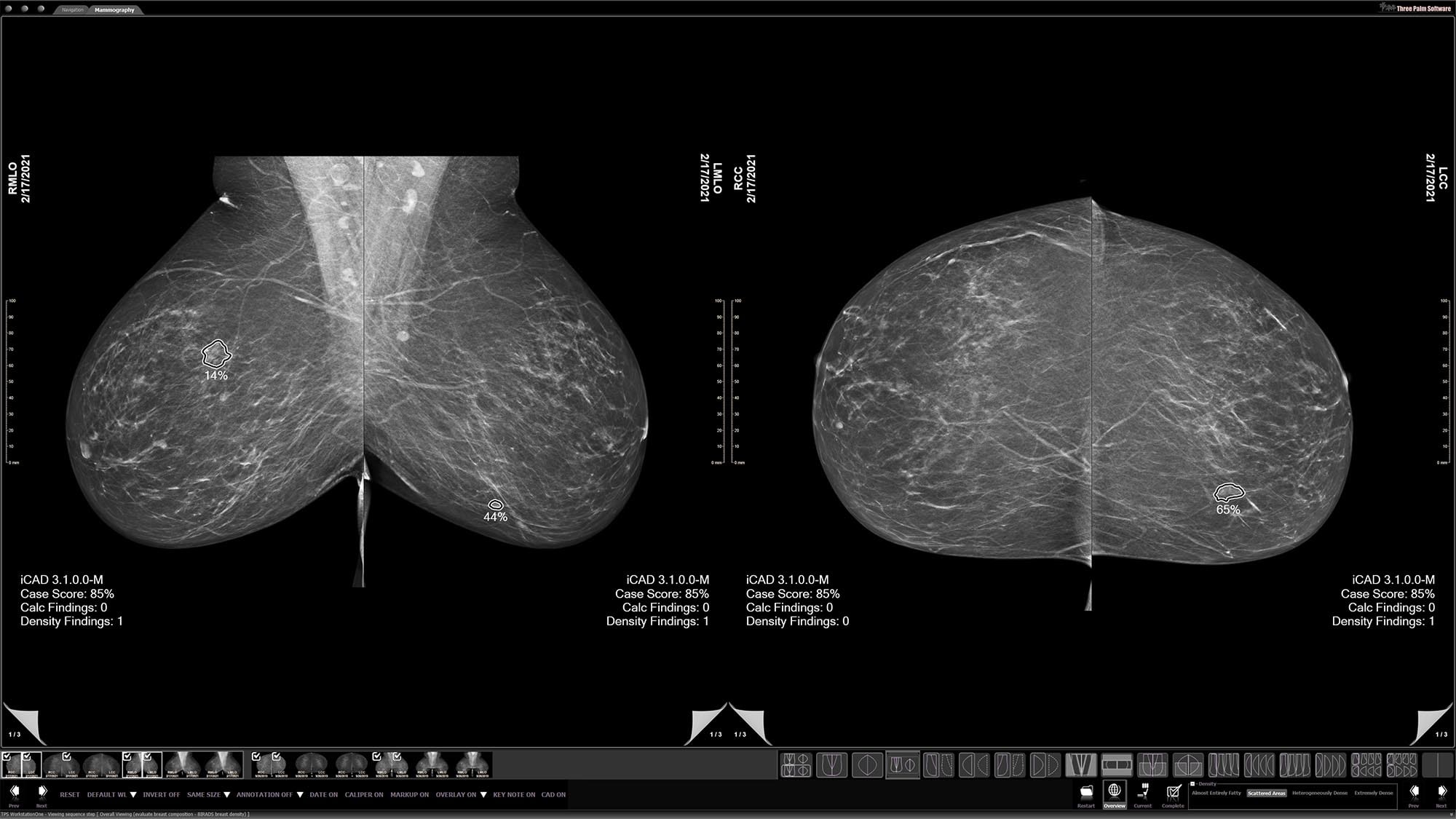
Density assessment
Automatically generates reports that adhere to BI-RADS® tissue density categories from mammograms to help provide consistent breast anatomy assessments.3

Improved accuracy when incorporating priors
The goal of incorporating priors is to evaluate lesion evaluation over time. The score may change based on the evolution or stability of the lesion. The overall accuracy of MammoScreen improves when incorporating priors.4
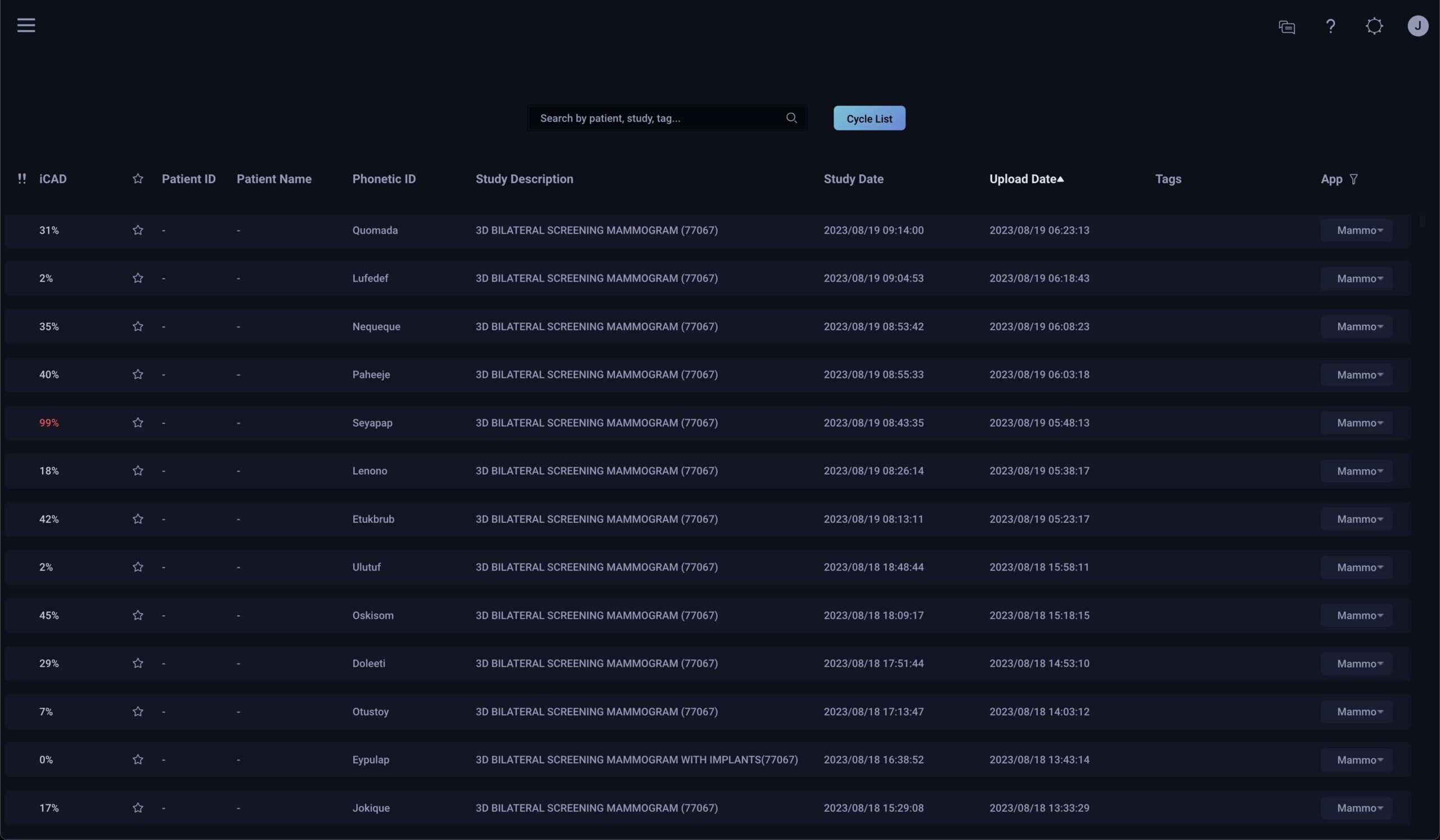
worklist flagging
Automatically flags patient cases where the algorithm showed a higher confidence of a malignant finding, helping providers to timely review screening 2D and 3D breast images.2
- Conant EF, Toledano AY, Periaswamy S, et al. Improving accuracy and efficiency with concurrent use of artificial intelligence for digital breast tomosynthesis. Radiology: Artificial Intelligence. 2019;1(4):e180096. https://doi.org/10.1148/ryai.2019180096
- Tempus Pixel is FDA-cleared (K203744) and CE marked; detection of lesions suspected of breast cancer is powered by iCAD’s profound AI (K203822). Arterys Inc is the manufacturer of Tempus Pixel, excluding any third party components described in this list. iCAD is the manufacturer of ProFound AI.
- Breast density assessment is powered by iCAD’s ProFound AI (K203822). iCAD is the manufacturer of the Breast density assessment.
- Tempus Pixel is FDA-cleared (K203744) and CE Marked. Therapixel is the manufacturer of MammoScreen® (K211541) which incorporates priors in its cancer detection analysis. Arterys Inc is the manufacturer of Tempus Pixel, excluding any third party components described in this list.