-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
02/14/2025
Q&A: Exploring the implications of CHIP on oncology patient care
In this Q&A, Dr. Ezra Cohen, Chief Medical Officer of Oncology, discusses key considerations and implications of clonal hematopoiesis of indeterminate potential (CHIP) for oncologists, focusing on its impact on genetic testing, diagnosis, and treatment decisions to help clinicians provide the best possible care for patients.
Authors
Ezra Cohen, MD
Chief Medical Officer of Oncology, Tempus

Chief Medical Officer of Oncology, Tempus

What is CHIP, and why is it significant for oncologists to be aware of? |
| Ezra Cohen, MD: CHIP stands for clonal hematopoiesis of indeterminate potential. Essentially, it refers to a collection of blood cells that have acquired genetic changes over time. These mutations are not inherited; and many develop as a person gets older. By the time someone reaches 70, there’s about a 10% chance they have a CHIP alteration in their blood cells.1As labs develop their capabilities, adding CHIP detection can be helpful because CHIP can complicate genetic test results. When using next-generation sequencing (NGS) to look for cancer mutations, CHIP alterations can make it appear as if they are cancer-related when, in fact, these alterations may be present due to factors such as age, prior cytotoxic chemotherapy, among others. |
How can CHIP mutations be mistakenly interpreted as tumor-derived mutations in solid tumors? |
| Ezra Cohen, MD: CHIP mutations often occur in specific genes, most of which are also targeted by NGS for their oncogenic implications. This overlap can potentially lead to misinterpretation, where CHIP mutations are mistaken for tumor-derived somatic mutations.For example, a mutation in the TP53 gene might appear as if it is driving cancer when, in reality, it is simply a byproduct of CHIP. Other genes associated with CHIP include KRAS, BRCA2, ATM, IDH1, and IDH2—all of which are potential targets for cancer therapies. If these mutations are related to CHIP rather than a tumor, they could lead to false positives.2,3 |
How do variant allele frequencies contribute to the confusion between CHIP and true cancer-driving alterations? |
| Ezra Cohen, MD: Most CHIP mutations have low variant allele frequencies (VAF)—typically 2%4. When mutations are detected at these low VAF, it becomes especially challenging to determine whether they are cancer-driven somatic alterations or CHIP-related. Bioinformatic models can assist in distinguishing between the two, but without these tools, misinterpretation is a potential risk.One way to detect CHIP is by sequencing the buffy coat, the layer of white blood cells that sits atop the red cells after blood is centrifuged. By sequencing the DNA from this layer, oncologists can identify CHIP mutations in the white blood cells. In cases where sequencing is already done using a tumor-normal match approach, the buffy coat is often sequenced by default, which can help detect CHIP.At Tempus, we have developed bioinformatic models based on insights from thousands of patients who’ve undergone tumor-normal match sequencing. These models enable us to better differentiate likely clonal hematopoiesis mutations from true oncogenic alterations in certain of our tests, helping to reduce false positives. |
Given that CHIP is more commonly associated with hematologic malignancies, how should oncologists handle CHIP findings in the context of solid tumor management? |
| Ezra Cohen, MD: Patients with CHIP may have an increased lifetime risk of developing blood cancers. For example, if the VAF exceeds 10%, the annual risk of developing a hematologic malignancy is around 1%.5 While this risk is relatively low, it is still higher than the general population’s, so monitoring these patients is recommended.Additionally, CHIP mutations are linked to an elevated risk for cardiovascular diseases and death from cardiovascular causes.6Though no specific treatments are available for CHIP itself, the focus should be on overall health monitoring. Oncologists should remain vigilant and refer patients to specialized clinics when necessary. |
How would you advise physicians to integrate CHIP findings into their clinical practice without compromising the treatment plan? |
| Ezra Cohen, MD: The field of CHIP is evolving rapidly, and in the past 3-4 years, research has significantly expanded. While there is much ongoing investigation, oncologists should take steps to accurately interpret mutations with low VAFs to avoid mistaking them for oncogenic drivers.For example, if a lab has not conducted additional bioinformatic analysis or failed to sequence the buffy coat, physicians may inadvertently misinterpret low VAF findings as tumor-derived. To avoid this, oncologists should ensure that sequencing methods are robust and that they have access to accurate bioinformatic tools that, to the extent possible, can help differentiate CHIP mutations from true cancer drivers. |
1. Reed SC, Croessmann S, Park BH. CHIP happens: Clonal hematopoiesis of indeterminate potential and its relationship to solid tumors. Clin Cancer Res. 2023;29(8):1403-1411.
2. Hu Y, Ulrich BC, Supplee J, et al. False-positive plasma genotyping due to clonal hematopoiesis. Clin Cancer Res. 2018;24(18):4437-4443.
3. Jensen K, Konnick EQ, Schweizer MT, et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference.JAMA Oncol. 2021;7(1):107.
4. Uddin MM, Zhou Y, Bick AG, et al. Longitudinal profiling of clonal hematopoiesis provides insight into clonal dynamics. Immun Ageing. 2022;19:23.
5. Jaiswal S, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498.
6. Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation, and cardiovascular disease. J Mol Cell Cardiol. 2021;161:98-105.
-
04/02/2025
Development of a clinical algorithm to prognosticate response to immunotherapy
Discover how Tempus developed and deployed the Immune Profile Score (IPS)—a powerful algorithm that provides prognostic insights into patient outcomes following treatment with immune checkpoint inhibitors (ICIs)—in ~18 months. This case study highlights the AI-driven methodology, real-world validation, and the impact of IPS in precision oncology.
Read more -
03/25/2025
AI & ML in action: Unlocking RWD with GenAI through Tempus Lens
Discover how Tempus is equipping researchers with innovative AI solutions to fully leverage the potential of multimodal data. Gain insights from a panel of leaders across healthcare and life sciences as they discuss the impact of these advanced tools on delivering insights with speed.
Watch replay
Secure your recording now. -
03/11/2025
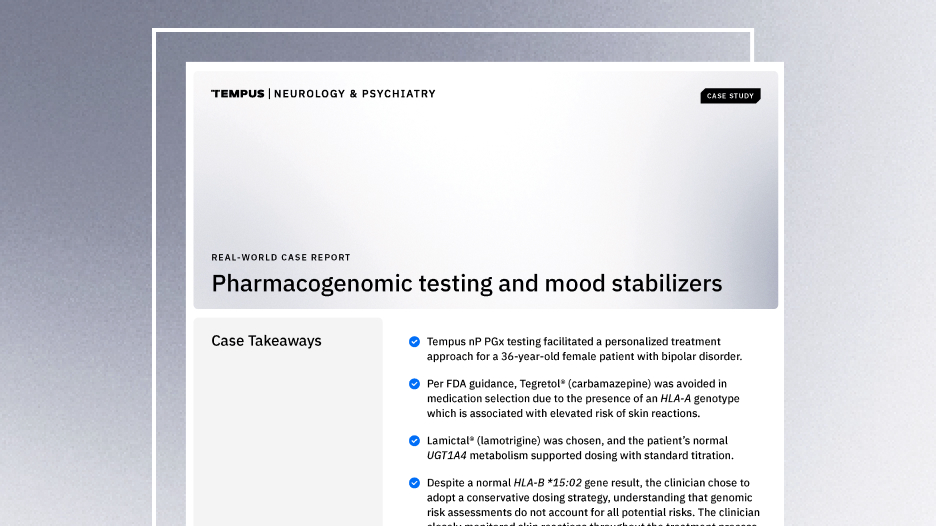
Case Report: Pharmacogenomic testing and mood stabilizers
This real-world case demonstrates how the Tempus nP pharmacogenomic test facilitated a personalized treatment approach for a patient with bipolar disorder.
Read more