-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
03/14/2025
Q&A: Navigating the future of IO: Biomarkers, combination therapies, and clinical strategies
In a recent webinar hosted by Tempus, industry experts gathered to discuss the latest in immuno-oncology (IO), where the cancer research landscape is ever-evolving due to the potential of emerging therapies.
Authors
Natalie Vokes, MD
Assistant Professor, MD Anderson Cancer Center

Timothy Taxter, MD
Executive Medical Director, Tempus

Douglas Palmer, PhD
Executive Director of Immuno-Oncology Translational Medicine, AstraZeneca

Assistant Professor, MD Anderson Cancer Center

Timothy Taxter, MD
Executive Medical Director, Tempus

Douglas Palmer, PhD
Executive Director of Immuno-Oncology Translational Medicine, AstraZeneca

Doctors Natalie Vokes, MD, Assistant Professor at MD Anderson Cancer Center, Timothy Taxter, MD, Executive Medical Director at Tempus, and Douglas Palmer, PhD, Executive Director of Immuno-Oncology Translational Medicine at AstraZeneca, recently spoke in detail about advancements and emerging trends in IO development. The discussion focused on the role of novel biomarkers and combination treatment strategies in enhancing the immune system’s response to cancers, as well as the significant developments that are helping to shape clinical trials and improve patient outcomes in IO. The conversation was moderated by Calvin Chao, MD, Sr. Vice President of Medical Affairs at Tempus.
Overview of the IO landscape |
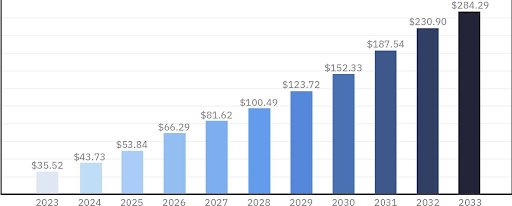
| Calvin Chao, MD: Today, there are nearly 4,000 active IO drugs in development, with one in six of all active drug development programs being IO-focused.1 The IO market is rapidly growing and is anticipated to continue, with the projected market value expected to exceed $280 billion by 2033.2 Amidst this growth, we’ve seen challenges and new regulatory rulings emerge.
IO market size from 2023 to 2033 (USD billion)2
|
Calvin Chao, MD: The FDA’s Oncologic Drugs Advisory Committee (ODAC) recently ruled against PD-1 inhibitors in HER2–, MSS gastric/GEJ adenocarcinoma with a PD-L1 expression <1. Do you foresee this being an unmet need in other disease spaces? Are there ongoing research efforts to identify biomarkers that outperform PD-L1 and TMB? If so, what does that look like? |
| Natalie Vokes, MD: The ODAC ruling reflects the complex landscape of biomarker approvals in immunotherapy. PD-L1, while a key biomarker, is not flawless and may not consistently identify responders across clinical trials, suggesting the need for disease-specific cutoffs. This may explain some of the heterogeneity we see regarding the outcome association, the trial results, and the approval landscape.
As research progresses – especially in areas with approved PD-1 inhibitors – integrating refined biomarkers into trial designs is crucial. Researchers have focused on single-feature biomarkers like PD-L1 and tumor mutational burden (TMB), which interact at the tumor-immune interface, where capturing the interplay between the tumor and immune system is essential. Countless efforts are underway to capture this, from leveraging novel sequencing technologies like single-cell, to AI-enhanced analyses of pathomics and radiomics, to sophisticated models that incorporate both. While it will be challenging to implement immediately, incorporating these approaches into clinical trials is essential for improving patient outcomes. |
|
“Researchers have focused on single-feature biomarkers like PD-L1 and tumor mutational burden (TMB), which interact at the tumor-immune interface, so capturing the interplay between the tumor and immune system is essential.” – Natalie Vokes, MD, Assistant Professor, MD Anderson Cancer Center |
Calvin Chao, MD: When should one incorporate biomarker considerations into drug development strategies? |
| Doug Palmer, PhD: Integration should begin in the preclinical phase and extend through the clinical phase. Investments in discovering novel biomarkers have increased, but the real challenge lies in deploying them effectively. In the non-IO space, targeted therapies like antibody-drug conjugates (ADCs) are being developed, and AI digital pathology-type approaches are being used to measure targets such as T-cell engagers or CAR-T targets. However, having the right target doesn’t necessarily guarantee success.
When considering combinations and targeted approaches with an IO agent, the industry’s approach has evolved and the broad labels are gone; the focus is now on finding a niche where drugs have the right benefit. This work often involves collaborating with different partners, like Tempus, where running biomarker studies in standard-of-care patients helps understand when patients are not responding to therapies and why. The process must be end-to-end — from research to development, and ultimately to deployment. |
Calvin Chao, MD: Apart from PDL1/TMB/MSI-H, what data do clinicians need to improve treatment selection? |
| Natalie Vokes, MD: Going forward, we need data that provides a readout of both tumor and immune biology, which should be captured both before treatment initiation and reassessed over time. This is where I believe circulating biomarkers and liquid-based assays are particularly promising, as they offer a more accessible biospecimen source for building long-term datasets. While there is much more to be learned from the tissue, technological advancements are helping enable more in-depth profiling of liquid biopsies, such as single-cell spatial and sub-single-cell spatial analyses. Also, the application of AI to digital hematoxylin and eosin (H&E) stains will allow for the extraction of more information from limited sequential sections, which would be invaluable.
I’m excited about the potential of combining detailed profiling on pretreatment biopsies using novel platforms and with investments in novel technologies applied to blood samples. This will help to assess treatment response dynamically, differentiate between various responder types and resistance mechanisms early on, and guide patients into tailored trial strategies. However, the cost of building these datasets poses a challenge to their realistic integration into clinical practice. |
Calvin Chao, MD: As we explore the potential of novel biomarkers, what platforms do you feel are particularly promising in these arenas? |
| Doug Palmer, PhD: AI pathology stands out as an encouraging tool. The ability to scan an H&E image and analyze immune infiltration or activation has existed for a while, but the challenge has been deploying these insights in clinical studies and practice. In clinical trial design, the focus is typically on whether patients respond to a specific therapy, rather than understanding why some patients don’t respond to standard-of-care treatments. A design framework that allows therapeutic interventions to be robustly evaluated and that is geared toward deployment would help to address this gap.
Foundational models are also generating excitement, with the ability to create clone-like images from RNA-seq data that can even fool pathologists. The key to effectively transitioning from discovery, to development, and eventually the deployment of novel biomarkers is making use of these new platforms from the outset in a way that will seamlessly integrate into the later stages of drug development. This requires investing in clinically relevant samples and real-world data (RWD) to understand what a successful program would look like and working backward from there. |
|
“The key to effectively transitioning from discovery, to development, and eventually the deployment of novel biomarkers is […] investing in clinically relevant samples and real-world data (RWD) to understand what a successful program would look like and working backward from there.” – Doug Palmer, PhD, Executive Director Head IO Translational Medicine, AstraZeneca |
Calvin Chao, MD: While PD-1/PD-L1 are well-established, they still aren’t perfect. Dr. Taxter, in what direction does the industry go from here to continue pushing the boundaries of immunotherapy? |
| Tim Taxter, MD: Simply put, we need more biomarkers. At Tempus, we recently developed and deployed Immune Profile Score (IPS), a novel biomarker designed to enhance the identification of patients who may benefit from immune checkpoint inhibitor (ICI) therapies. IPS leverages our RNA sequencing assay, xR, to maximize the understanding of immune functionality. It incorporates TMB and single-gene RNA features — including familiar ones like CXCL9 or PD-L1 — as well as novel Tempus signatures derived from single-cell sequencing.
Our clinical validation in a pan-cancer setting showed that the IPS signature outperformed known clinical biomarkers, offering strong stratification in the overall cohort. This validation was just the beginning of the journey — we’re now focusing on collaborating on future clinical development projects and considering how these insights fit into the evolving immunotherapy space, particularly in combination with other treatment strategies. |
Calvin Chao, MD: Dr. Vokes, is there a particular mechanism of resistance that you’re most concerned about/interested in? What should drug developers/researchers be doing to address this? |
| Natalie Vokes, MD: There has been a significant focus on predictors of immunotherapy response, but less on mechanisms of resistance, which is a critical unmet research need. Clinically, we often categorize resistance as intrinsic or acquired, considering the timing and whether it’s at an isolated site or more widespread, which can greatly inform treatment decisions. When we create those categories, I suspect we’re dealing with differentiation between cold tumors and non-cold tumors, which is particularly important. In non-cold tumors with acquired resistance, there’s much to learn about antigen loss, changes in T-cell phenotypes, and the role of myeloid and immunosuppressive populations that emerge later. |
Calvin Chao, MD: What has your experience been with primary and secondary disease resistance in the clinic? |
| Natalie Vokes, MD: It’s important to note that when we’re discussing immunotherapy combinations, the conversation has to involve both primary and secondary disease resistance. The goal for immunotherapy patients is long-term, durable disease control, which IO aims to provide. However, response rates don’t always reflect long-term control. For example, chemotherapy in combination with immunotherapy may improve initial response rates but doesn’t necessarily lead to an effective, long-lasting anti-tumor immune response.
Primary resistance and predictors of radiographic response are critical; we need to identify who will respond to treatment. If a patient has a high disease burden, we might opt for aggressive treatment, but this doesn’t guarantee an induced anti-tumor immune response. It’s essential to differentiate between combining non-overlapping treatments and augmenting immunotherapy, which becomes more apparent in acquired resistance settings over time. Durability is another key focus, beyond initial response rates, to ensure we’re not just achieving temporary remissions but fostering sustained responses with immunotherapy. |
Calvin Chao, MD: What combination therapies are you most excited about or see the most promise for? |
| Tim Taxter, MD: I’ve been closely following the integration of radiation work into the IO space, where there’s been increasing focus on the timing of radiation and its effective combination with IO therapies. Understanding the biomarkers related to resistance and immune suppression is critical, as they can be quite different from biomarkers like PD-L1. There is also a lot of work being done on cancer-specific markers such as aneuploidy, which could play a key role in these novel radiation therapy trials. This area has historically lacked biomarker research in radiation oncology, so I’m eager to see how it evolves with the introduction of new therapies.
Natalie Vokes, MD: I’m excited about the clinical trial activity around LAG-3, which shows promise in melanoma and may extend to other cancer types. Checkpoint-based strategies are advancing to the frontline, and there’s potential for identifying patient populations who could benefit from additional drugs beyond PD-1 inhibitors. I anticipate that a small but significant percentage of patients will respond to these strategies, and biomarker research will be key in identifying them. Additionally, bispecifics are showing early positive data, offering different combination antibody approaches within a single molecule. Other promising areas include cytokine-based strategies and cellular-based therapies that could more effectively alter the tumor immune microenvironment and enhance immune responses beyond what ICIs alone can achieve. Doug Palmer, PhD: It’s not just about trying any combination; it’s more about understanding the timing and how that impacts efficacy. For example, we’ve learned that sequential combinations might not be beneficial, and timing can be quite critical, especially with checkpoint blockade and small molecule inhibitor studies. What’s intriguing is the epidemiology of resistance — understanding which patients are not responding and why. For instance, antigen processing wasn’t as significant a resistance pathway as we thought, which was surprising. I agree with Natalie about bispecifics and the resurgence of ADCs. I’ve spent 20 years in cell therapy and have seen the durable responses it can achieve, despite the challenges in production and managing toxicities. We’re now thinking about how to model ADCs with other targets, like poly (ADP-ribose) polymerase (PARP) inhibitors or even cell therapy, to de-risk investments and build better models that are more clinically actionable. |
For more insights on IO development and to learn how Tempus is providing actionable and cutting-edge services for life sciences partners advancing immunotherapies, explore our sequencing solutions.
*Note: content edited for clarity.
Please note that the content in this document has been revised for clarity and conciseness. Some language and formatting may have been adjusted to enhance readability while preserving the original meaning and intent of the discussion.
1. Citeline. Pharma R&D Annual Review 2024.
2. Based on Citeline clinical trial query, pulled on October 25, 2024
-
04/02/2025
Development of a clinical algorithm to prognosticate response to immunotherapy
Discover how Tempus developed and deployed the Immune Profile Score (IPS)—a powerful algorithm that provides prognostic insights into patient outcomes following treatment with immune checkpoint inhibitors (ICIs)—in ~18 months. This case study highlights the AI-driven methodology, real-world validation, and the impact of IPS in precision oncology.
Read more -
03/25/2025
AI & ML in action: Unlocking RWD with GenAI through Tempus Lens
Discover how Tempus is equipping researchers with innovative AI solutions to fully leverage the potential of multimodal data. Gain insights from a panel of leaders across healthcare and life sciences as they discuss the impact of these advanced tools on delivering insights with speed.
Watch replay
Secure your recording now. -
03/11/2025
Case Report: Pharmacogenomic testing and mood stabilizers
This real-world case demonstrates how the Tempus nP pharmacogenomic test facilitated a personalized treatment approach for a patient with bipolar disorder.
Read more