-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
ENROLL NOW
Tempus’ patient-derived organoid screens
Evaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
08/20/2024
Q&A: The evolving role of antibody-drug conjugates in oncology
Dr. Kate Sasser from Tempus led a discussion on the potential of antibody-drug conjugates (ADCs), their challenges in targeting tumor antigens, and the future of cancer treatment with Dr. Daniel Johnson, Dr. Funda Meric-Bernstam, and Dr. Kellogg Parsons.
Authors
Kate Sasser, PhD
Chief Scientific Officer, Tempus

Daniel Johnson, MD
Director, Precision Cancer Therapies Program, Ochsner MD Anderson Cancer Center

Funda Meric-Bernstam, MD
Chair, Department of Investigational Cancer Therapeutics (Phase I Program), MD Anderson Cancer Center

Kellogg Parsons, MD, MHS, FACS
SVP, Clinical Development, MBrace Therapeutics

Chief Scientific Officer, Tempus

Daniel Johnson, MD
Director, Precision Cancer Therapies Program, Ochsner MD Anderson Cancer Center

Funda Meric-Bernstam, MD
Chair, Department of Investigational Cancer Therapeutics (Phase I Program), MD Anderson Cancer Center

Kellogg Parsons, MD, MHS, FACS
SVP, Clinical Development, MBrace Therapeutics

Q&A: The evolving role of antibody-drug conjugates in oncology
The oncology field is rapidly advancing with the development of antibody-drug conjugates (ADCs), offering new hope for precision medicine. Dr. Kate Sasser from Tempus led a discussion with Dr. Daniel Johnson, Dr. Funda Meric-Bernstam, and Dr. Kellogg Parsons on the potential of ADCs, the challenges in targeting tumor antigens, and the future of cancer treatment.
Overview of antibody-drug conjugates (ADCs) |
| Kate Sasser, PhD: ADCs are a hot topic, with significant excitement around their development. There’s a notable increase in preclinical activity, and we anticipate many new therapeutics advancing to later stages. One reason for the excitement is the higher technical and regulatory success rates for ADCs compared to other oncology assets. However, the field is becoming crowded, especially in common indications like breast and lung cancer. We’ll discuss strategies to move beyond the most common targets. ADCs are defined by three key characteristics: the tumor target, the payload, and the linker technology. We’ll explore each area’s potential for innovation. |
How ADCs fit into the precision medicine landscape
Kate Sasser, PhD: Dr. Johnson, how do you approach matching the right patient to the right therapy with ADCs? |
| Daniel Johnson, MD: Generally, we see the objective response rates for targeted therapies, especially when patients are identified through markers on next-generation sequencing (NGS) or IHC, often exceeding that for immune checkpoint inhibitors due to the relative lack of precise biomarkers for immunotherapies. With ADCs, the identification of target antigens via biopsies currently tends to be based on IHC and not molecular sequencing tests. This can become challenging due to tissue constraints, especially if NGS tests are also needed. Adding an IHC test for each new ADC option further complicates matters. Precision medicine is critical for optimizing therapeutic outcomes, requiring innovative approaches to maximize diagnostic yield with minimal tissue usage. |
Kate Sasser, PhD: Dr. Meric-Bernstam, given the challenge of limited tissue and the increasing number of targets, how do you think we will approach precision medicine beyond IHC? |
| Funda Meric-Bernstam, MD: Over recent years, genomically informed therapies have shown significant success, with formerly non-actionable targets like KRAS becoming actionable. ADCs represent the next wave. Initially, many ADCs lacked biomarker selection for approval, prompting discussions on the relevance of target expression for drug efficacy. Data from DESTINY-PanTumor02, which I led, demonstrates that potent-payload ADCs with tumor-agnostic activity can achieve clinical efficacy across various epithelial tumors, which may justify IHC testing for multiple cancer types. Current ADC trials often require several slides for each IHC test, posing challenges for patients with limited tissue, as Dr. Johnson mentioned. How might we limit the number of patients we need to screen to find patients with positive IHC results to accrue? RNA offers additional advantages, potentially facilitating multiplex testing strategies essential for efficient marker identification. As well, are there additional biomarkers that can help us determine sensitivity to the ADC payloads, or associated with other ADC mechanisms? Comprehensive testing is likely to be invaluable in better understanding these biomarkers, especially since comprehensive RNA sequencing is being done more and more already, as we believe RNA provides a better approach for fusion detections. |
Developing the next generation of ADCs
Kate Sasser PhD: Dr. Parsons, in running clinical development at a smaller biotech, how do you approach new targets? What will be most impactful in advancing the field towards new targets of interest? |
| Kellogg Parsons, MD, MHS, FACS: We aim to enrich intent-to-treat populations with those most likely to respond to the drug. Dr. Meric did groundbreaking work in the DESTINY trial, looking at HER2 expression in a tumor-agnostic space. The challenge with a new target is determining the threshold of expression needed for a response – these thresholds may be very low, very high, or somewhere in the middle. Approved agents for solid tumors may or may not have companion diagnostics. My approach focuses on identifying and studying a new target’s expression to find responsive patients, considering the regulatory component for companion diagnostics.
For developing new targets using emerging technologies like RNA expression, there are two approaches:
Funda Meric-Bernstam, MD: New ADCs are entering clinical trials at a challenging time when important decisions need to be made at trial initiation. As mentioned, if you believe the target is frequent enough, it makes sense to have an unselected patient population in the early phases, such as during dose escalation. However, if it’s a rare target, not having a selected strategy doesn’t help the patient or the program because the likelihood of efficacy will be diminished. Having as much data about expression before initiating the trial is important for designing the most effective clinical trial.
|
Kate Sasser PhD: How do you think about layering in real-world data alongside more curated, deeper datasets? |
| Kellogg Parsons, MD, MHS, FACS: When you incorporate real-world data, you can start answering clinically relevant questions. For example, is your biomarker expression prognostic? Is it tied to a particularly lethal variant of the disease? This provides the first piece of information.
Predictions are trickier if you’re working with a target that’s never been drugged before. Without therapies directed at your target, you won’t have direct real-world data on whether knowledge of this target will inform treatment strategies. However, you can potentially access indirect data. For example, you could see if expression levels of your target vary with different lines of therapy. Is it expressed more or less with each subsequent line? Is your target co-expressed with other better known targets? You can tie this to clinically relevant outcomes to inform your strategy and approach, including potential combination therapies.
|
Overcoming mechanisms of resistance
Kate Sasser PhD: What about mechanisms of resistance — how might we overcome them? |
| Funda Meric-Bernstam, MD: ADCs have various potential resistance mechanisms. One is the loss of the target, where a potent payload eliminates target-expressing clones, leading to lower or non-expressing cells. This varies with different ADCs. In diseases like HER2-positive breast cancer, we need routine checks to ensure continued expression.
Another category is payload resistance. For example, mutations in the gene encoding topoisomerase can confer resistance to certain ADCs, though this is rare. Liquid biopsies may help monitor this, as some panels cover these mutations. Other resistance mechanisms include modulation of apoptotic machinery and upregulation of survival signaling. Additionally, there are ADC-related mechanisms such as drug trafficking and lysosomal function changes. Comprehensive profiling before and after treatment, especially in academic studies, will help us understand and address these resistance mechanisms more effectively.
|
Kate Sasser PhD: How do sequential ADC therapies factor into this? What information is useful to you when considering sequential ADC therapies, and how might we be designing trials to provide this information? |
| Daniel Johnson, MD: Clinically, we’re not yet able to identify mechanisms of resistance for ADCs and base decisions on them. The only way to identify that is prospectively in clinical trials. It’s easier to establish a prognosis with real-world data, but proving a marker is predictive is challenging. It requires many patients with biopsies and analyses to discern what’s predictive. Post-progression biopsies, which are challenging to obtain in trials, offer one approach to detect potential mechanisms of resistance.
Kellogg Parsons, MD, MHS, FACS: Another crucial factor is toxicity, as patient safety is always our priority. When designing or sequencing an ADC that follows an ADC already established as the standard of care, it’s important to consider the payloads for toxicity and the potential for cumulative toxicity. Patients previously exposed to a particular agent might be more sensitive to its cumulative toxic effects if given the same agent again.
|
Optimizing clinical trial design
Kate Sasser PhD: Could you comment on the clinical trial landscape and what’s important in building the right studies and finding the right patients? |
| Funda Meric-Bernstam, MD: This is an exciting time with many drugs in development. ADCs have demonstrated clinical efficacy even in early stages, emphasizing the importance of recognizing early activity. Designing trials that ensure appropriate patient selection while considering the competitive landscape is crucial. It’s essential to gather data on whether target expression sufficiently impacts selection in early development. This can be done through selective or dichotomized methods to enrich the efficacy signal as standards rise. As drugs improve, study designs must address the specific patient populations targeted. |
Kate Sasser PhD: How can we maximize data from early trials with small numbers and heterogeneous patient populations? |
| Daniel Johnson, MD: Ideally, in early-phase studies, more tissue is better, though it’s not always feasible logistically. Advocating for biopsies in patients is crucial to gather ample tissue for analysis. When upfront patient selection isn’t feasible due to novel targets, data is needed to establish cutoffs for IHC and its correlation with RNA expression.
Enhanced liquid biopsies could prospectively provide more data from patients. AI will play a critical role in biomarker discovery within clinical trials, sifting through extensive data from biopsies, next-generation sequencing, bulk RNA expression, IHC, and multiplex IHC, particularly in immunotherapy trials.
|
Kate Sasser PhD: Dr. Parsons, how should we approach combination therapies, especially involving immune therapies or TKIs? |
| Kellogg Parsons, MD, MHS, FACS: I consider combination approaches in two main aspects. First, does it make mechanistic sense to combine the agent with another? Is there a valid hypothesis? Second, does it fit within the landscape of available agents and potential partners? The combination must be grounded in both logical reasoning and the compatibility between the novel agent and existing drugs. A notable example is Enfortumab Vedotin combined with Pembrolizumab for advanced bladder cancer, marking a paradigm shift in therapy. |
Kate Sasser PhD: Do you believe ADCs, particularly those with chemotherapy payloads, will remain effective when combined with ICIs? |
| Daniel Johnson, MD: I think ADCs and IO combinations are promising due to their ability to induce immunogenic cell death and influence the immune microenvironment within tumors. Predicting effective drug combinations with IO agents and identifying responsive patient profiles pose significant challenges. RNA expression profiling, traditionally used for tumor targets, now aids in immune analysis by capturing both tumor cells and the tumor immune microenvironment in biopsies. However, sorting through these profiles to distinguish tumor cells from immune components and correlating this with treatment responses remains a formidable challenge. |
Looking ahead: The future of ADC development
Kate Sasser PhD: Which area do you believe is the most prime for disruption? |
| Daniel Johnson, MD: What excites me most in this space is probably based on the Enfortumab Vedotin and Pembrolizumab data. I’m particularly excited about combining it with IO, as I’ve mentioned before. While AI is often touted as the next big solution for solving these issues, I believe that using it in clinical trials for biomarker discovery will yield much better answers regarding patient selection for later-phase clinical trials. So, I’m very excited to see how that technology can enhance not just ADCs but all cancer clinical trials.
Kellogg Parsons, MD, MHS, FACS: I completely agree — I’m most excited about AI technologies. I think they have the opportunity to show us the way forward. I’m also excited about blood-based biomarkers. We’ve discussed the challenges of using IHC to quantify tumor expression. From my perspective, if I had to identify one of the most challenging bottlenecks, especially in early-phase trials but in any phase trial, it’s the availability of tissue that slows down progress. This also excludes patients in some cases who might otherwise not be excluded if we were able to use blood-based biomarkers. Funda Meric-Bernstam, MD: There is so much to be excited about it’s hard to pick just one. However, as we personalize patient selection, I believe exploring the development of new payloads could significantly extend the technology.
|
For further insights on ADCs and precision medicine, and to understand how Tempus is contributing to this field, access our white paper and learn about our role in advancing cancer care.
*Note: content edited for clarity.
Please note that the content in this document has been revised for clarity and conciseness. Some language and formatting may have been adjusted to enhance readability while preserving the original meaning and intent of the discussion.
-
12/11/2025
Driving enterprise value with RWD
Hear from biotech CEOs and VC leaders as they discuss how real-world data can inform strategic decision-making in biotech companies.
Watch replay -
11/11/2025
A new era of biopharma R&D: The TechBio revolution—realities and the next frontier
Join Tempus and Recursion leaders to explore their strategic TechBio partnership. Learn how they use AI and supercomputing with petabytes of data to accelerate drug discovery and development. See the impact on biopharma R&D's future.
Watch replay -
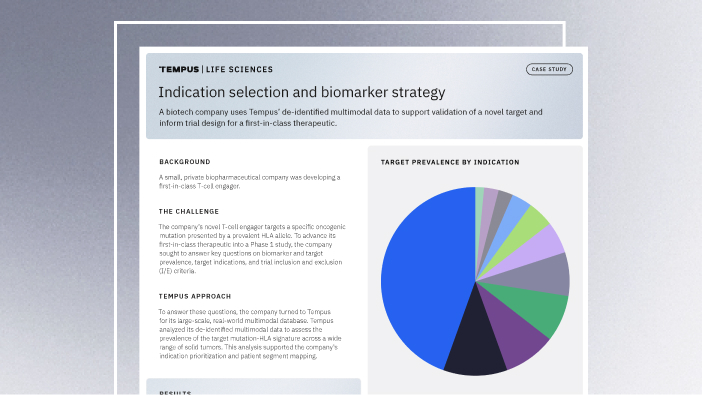
11/14/2025
Validating a novel target and informing trial design for a first-in-class therapeutic
Discover how a biopharma company used Tempus’ de-identified multimodal data to support validation of a novel target and inform trial design for a first-in-class therapeutic.
Read more