-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
Enroll now
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications. Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
RESOURCES
Document Library
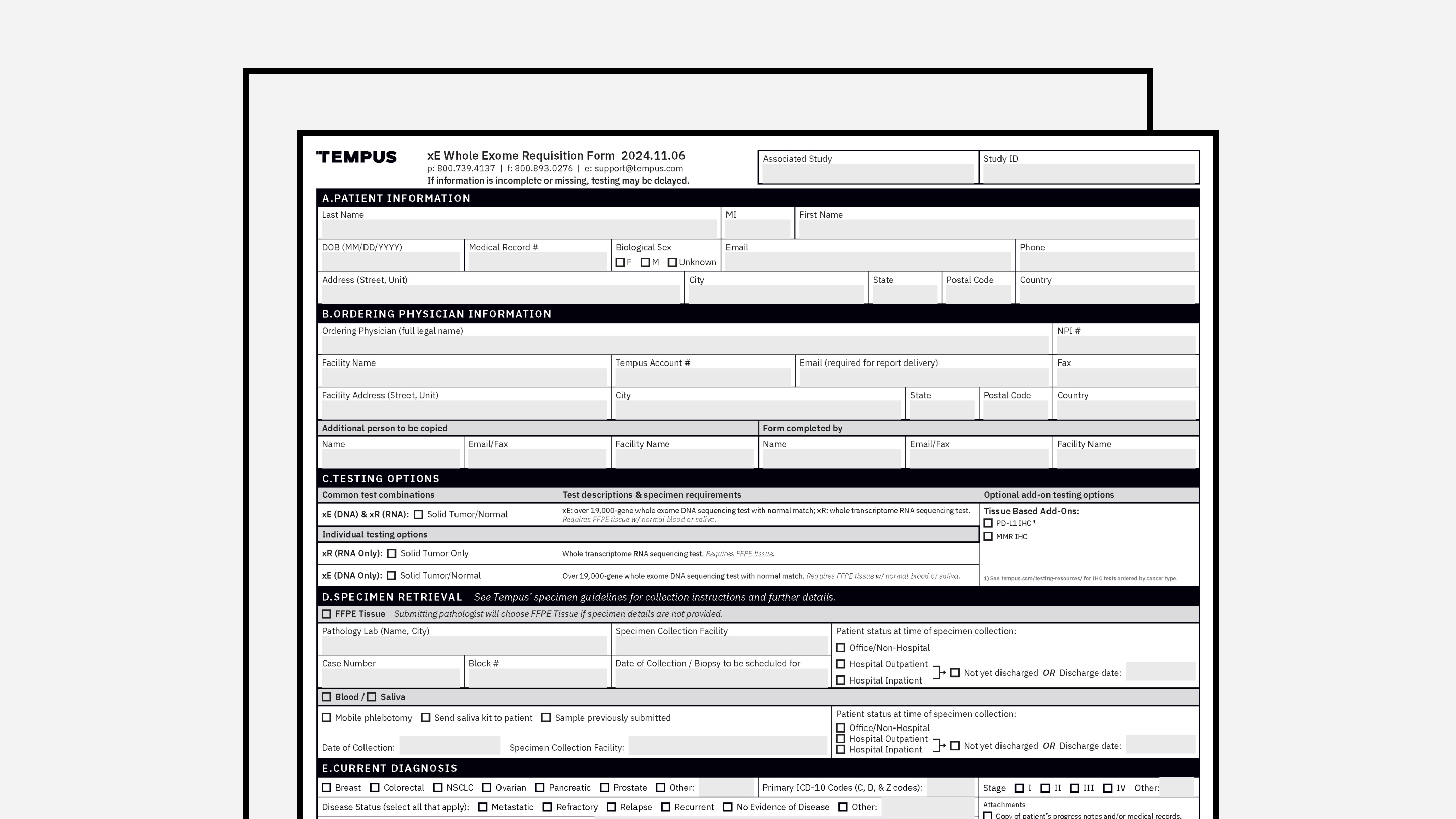
REQUISITION FORMS
-
REQUISITION FORMS
Requisition Form (Standard)
Tempus’ frontline order requisition form, without patient consent.
-
REQUISITION FORMS
Requisition Form (NYS)
Tempus’ frontline order requisition form, New York State specific.
-
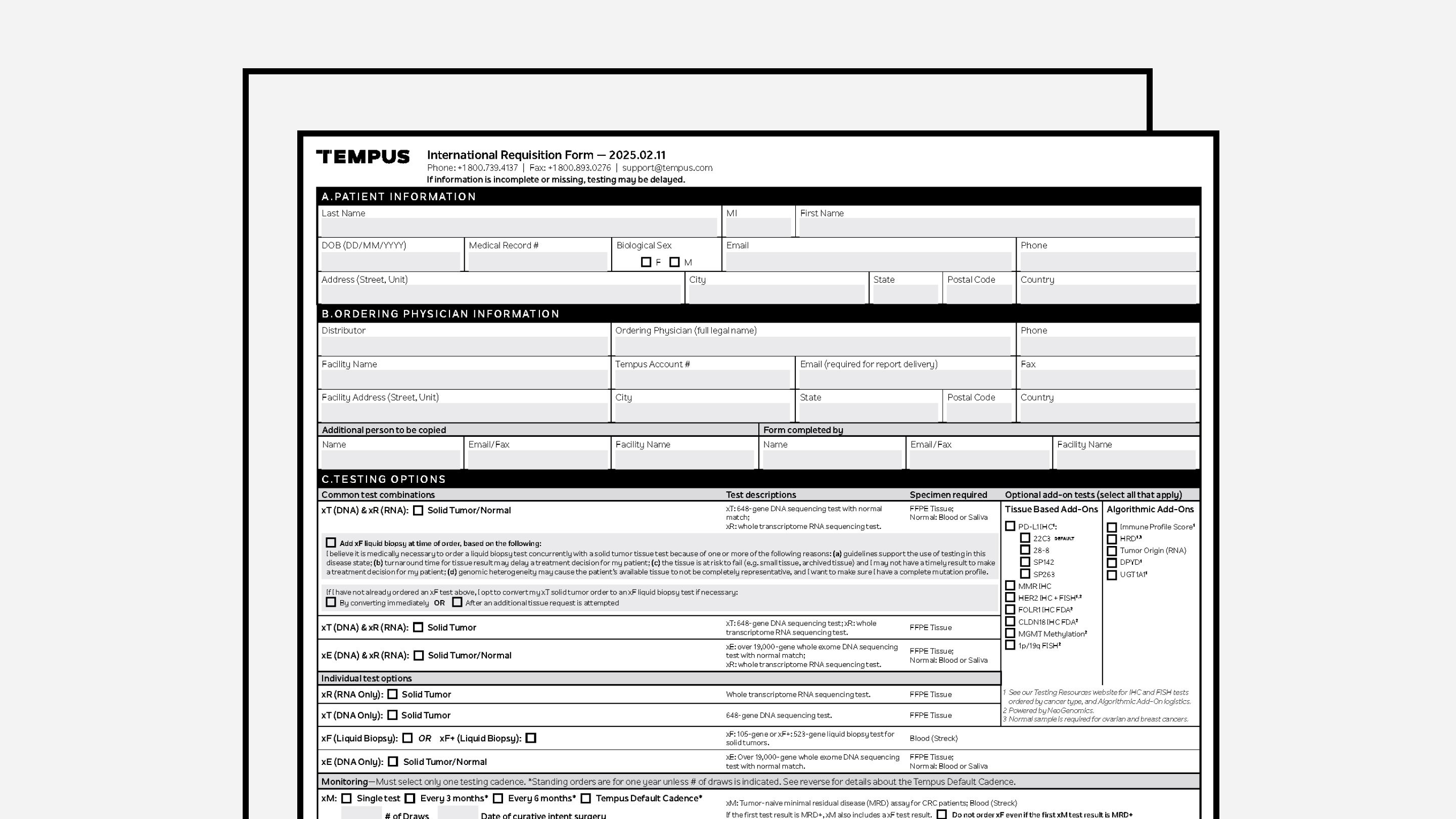
REQUISITION FORMS
Requisition Form (International)
Tempus’ frontline order requisition form, for international use.
-
REQUISITION FORMS
De-Identified Requisition Form (International)
A de-identified version of Tempus’ frontline order requisition form, for international use.
-
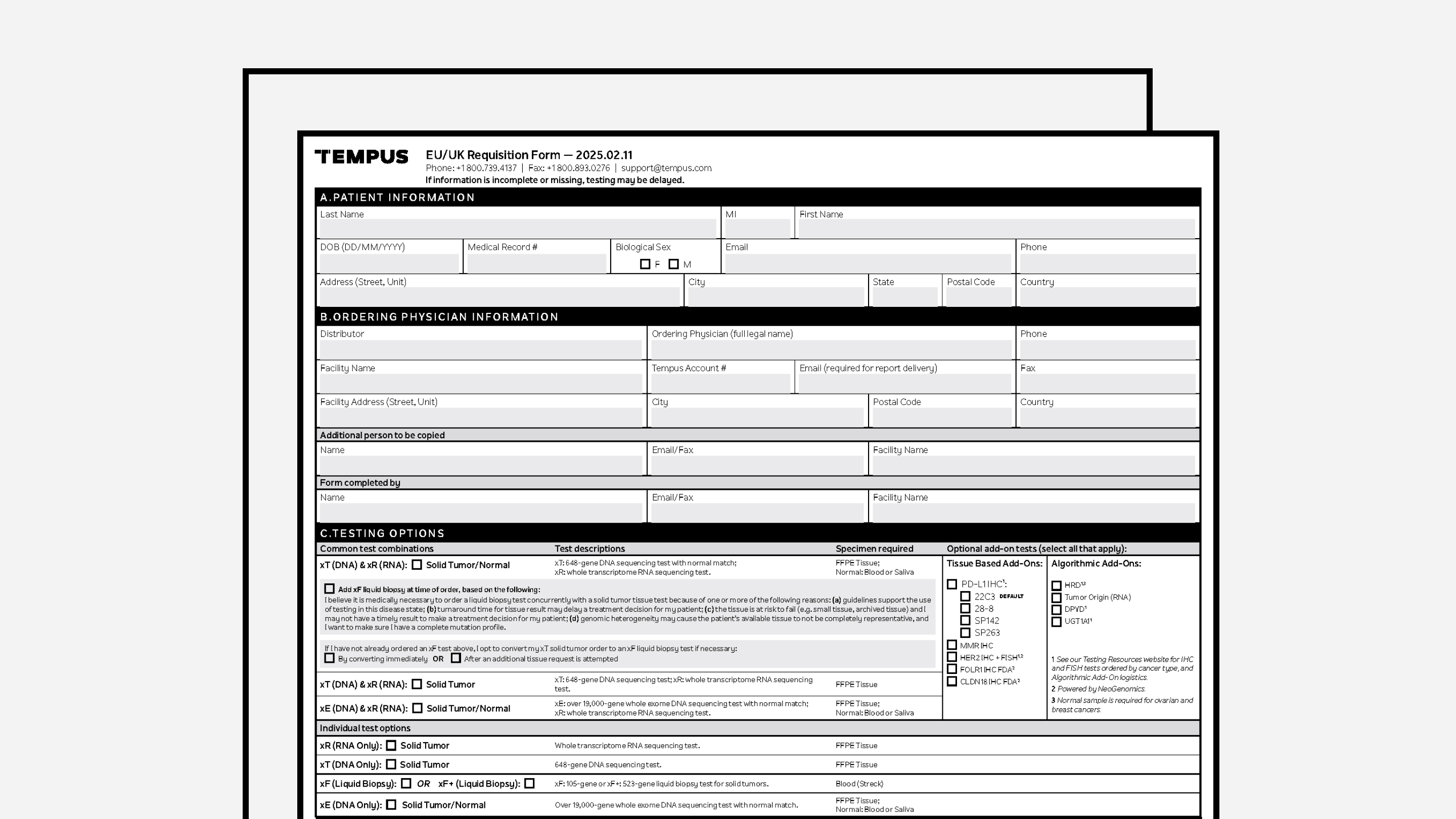
REQUISITION FORMS
De-Identified Requisition Form (EU-UK)
A de-identified version of Tempus’ frontline order requisition form, for EU/UK use.
-
REQUISITION FORMS
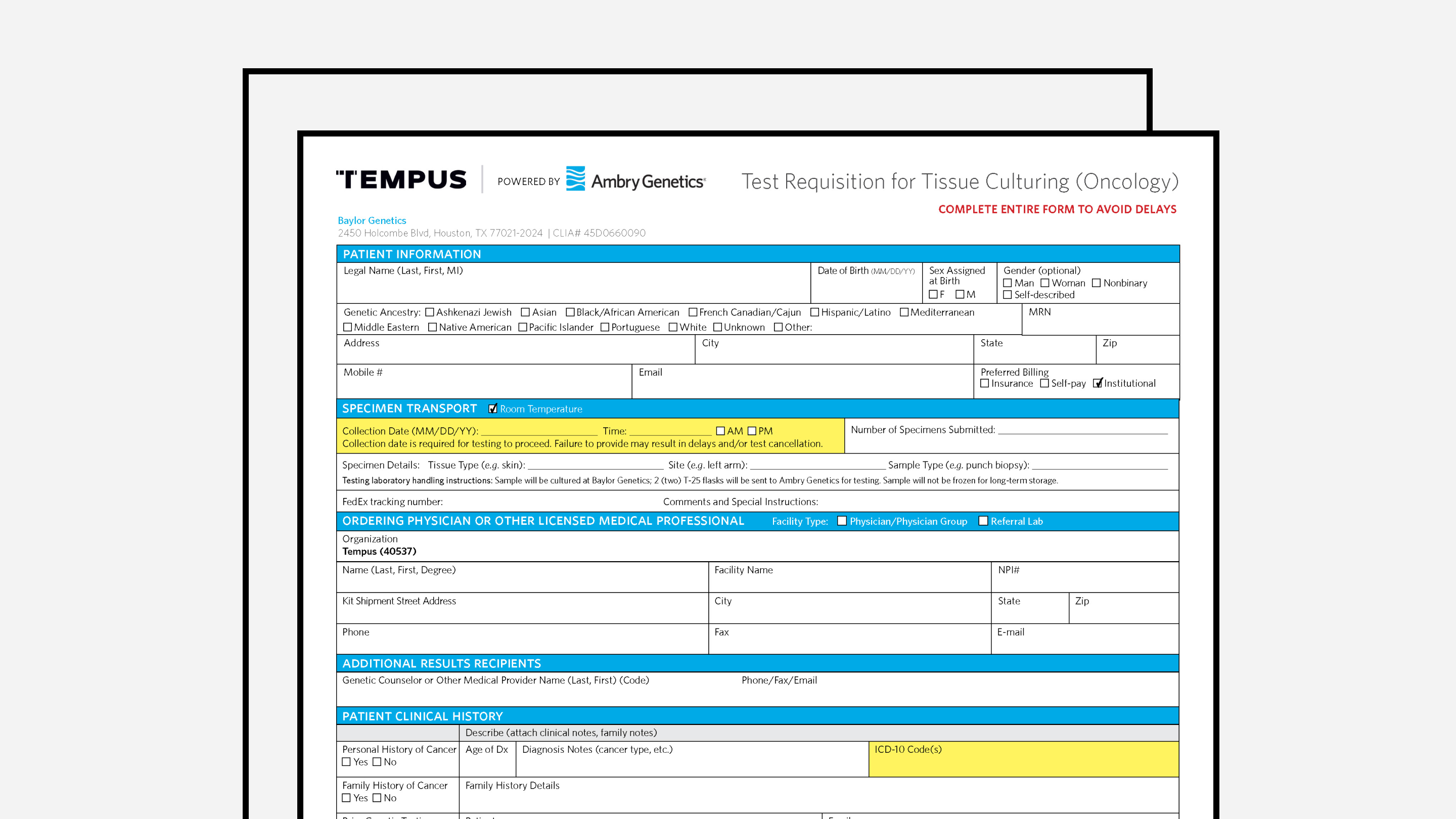
Hereditary Requisition Form (xG powered by Ambry Genetics®)
Tempus’ hereditary cancer requisition form.
-
REQUISITION FORMS
Requisition Form (xG Supplement)
A form containing supplemental information required for Tempus’ xG Hereditary Cancer Panel.
-
REQUISITION FORMS
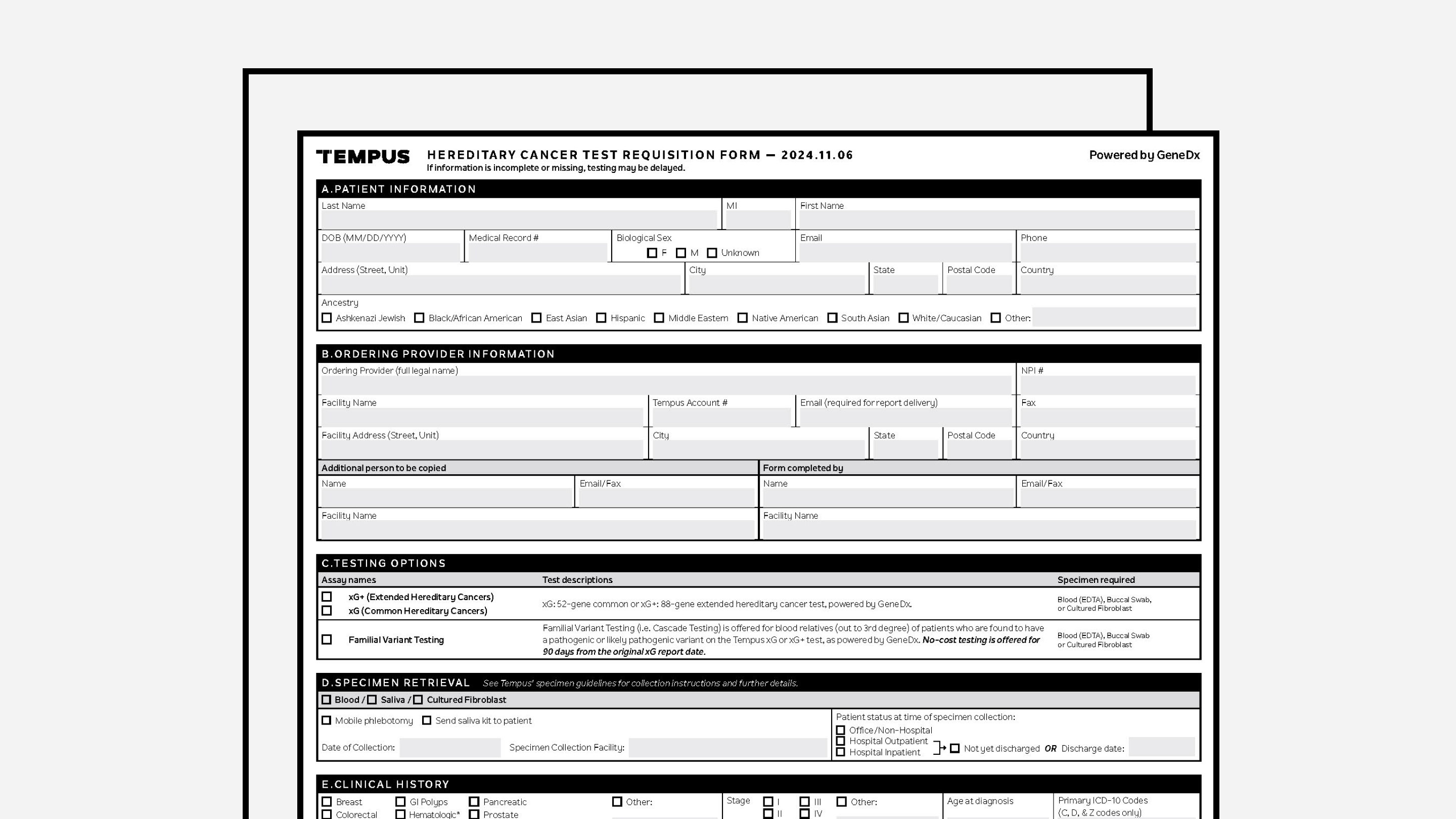
Hereditary Requisition Form (powered by GeneDX)
Tempus’ hereditary cancer requisition form.
-
REQUISITION FORMS
Requisition Form Guide
A guide designed to help you complete Tempus’ frontline requisition form.
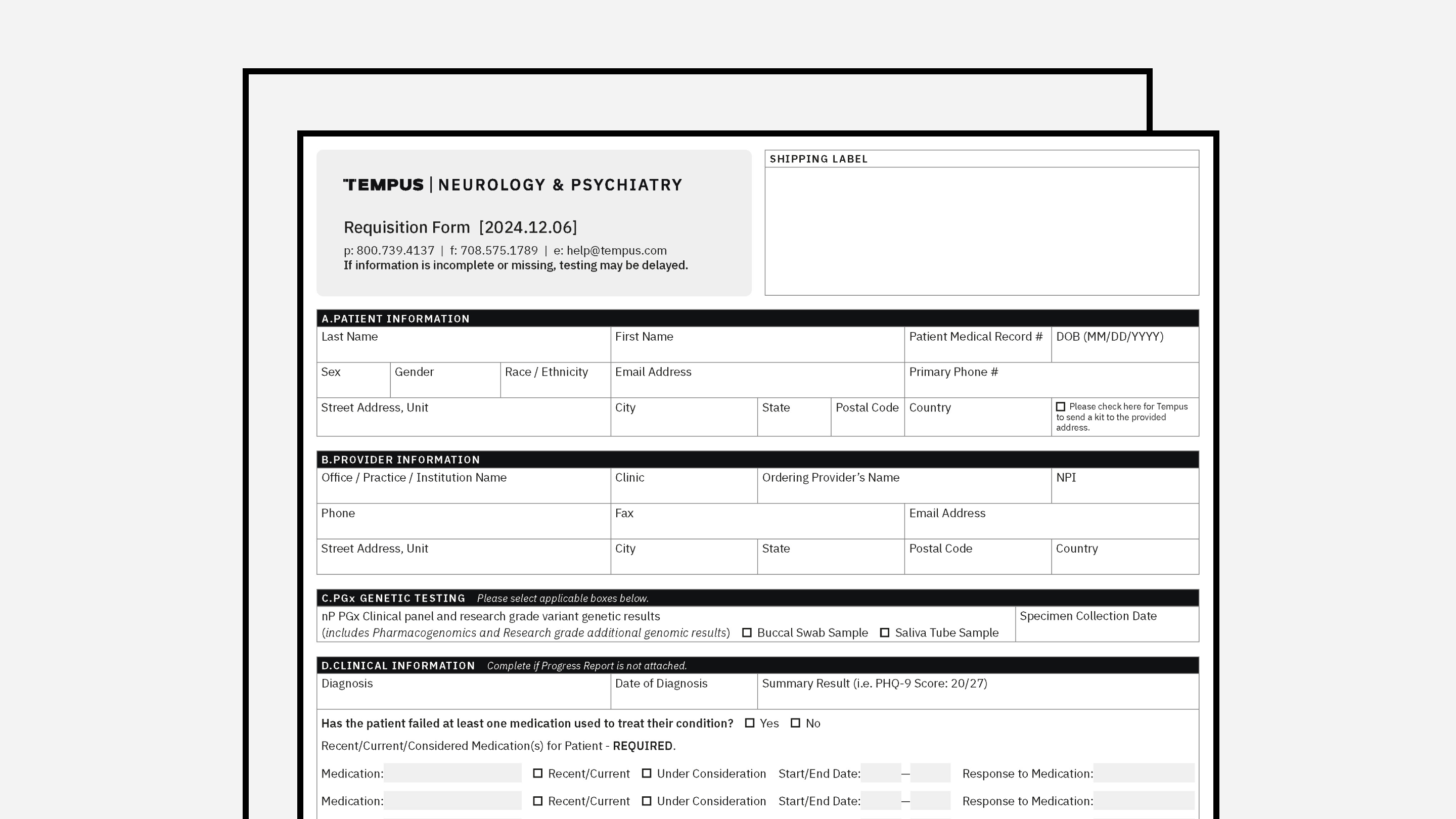
PATIENT FORMS
-
PATIENT FORMS
Patient Consent Form
Accompanying patient consent form to the frontline requisition form (only applicable in relevant states).
-
PATIENT FORMS
Patient Consent Form (NYS)
Accompanying patient consent form to the frontline requisition form (only applicable in New York State).
-
PATIENT FORMS
Notice And Authorization for Health Records
Tempus’ notice and authorization for health records form.
-
PATIENT FORMS
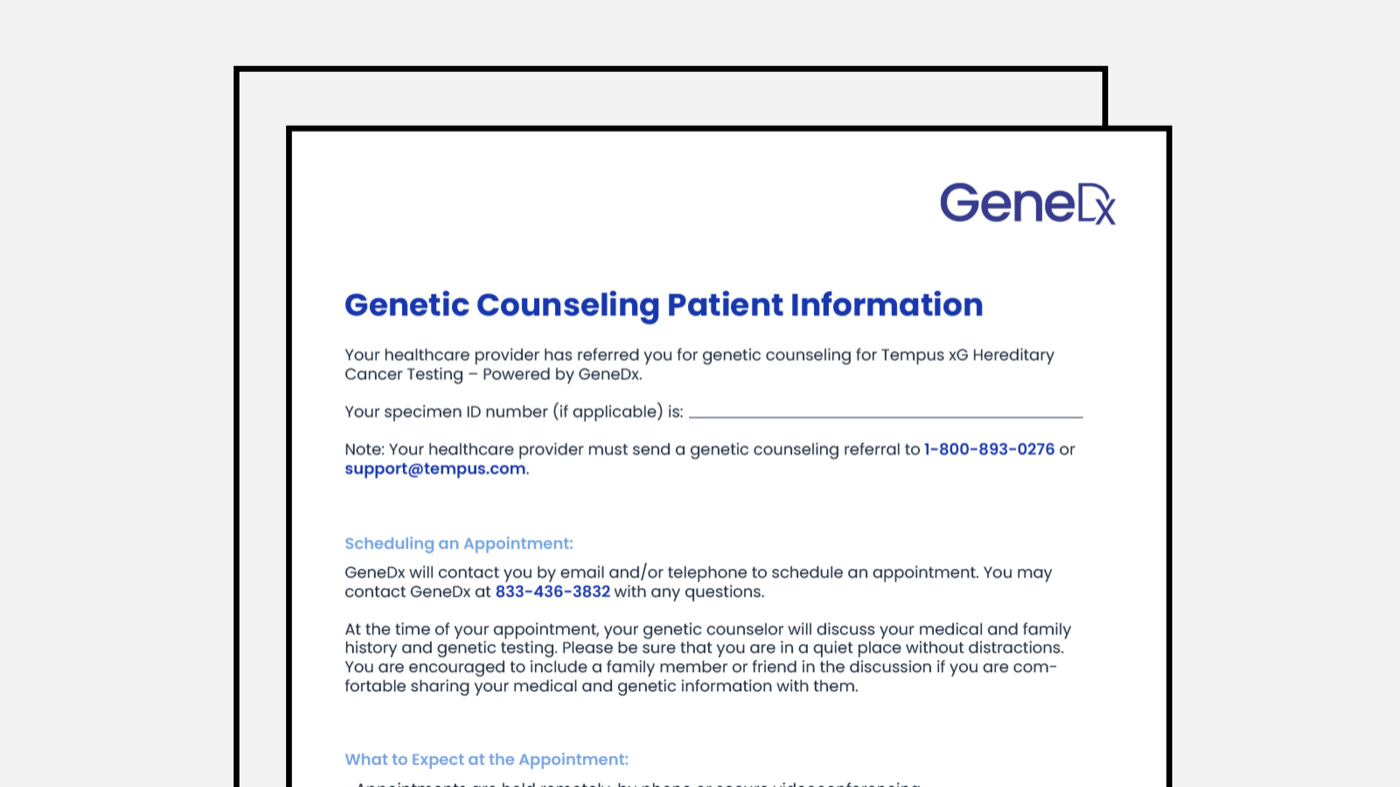
GeneDx xG Genetic Counseling Patient Scheduling Information
Genetic Counseling Patient Scheduling Information
-
PATIENT FORMS
Tempus nP Digital Patient Brochure (English)
Tempus’ patient information brochure for Neurology & Psychiatry.
-
PATIENT FORMS
Patient Consent Form (International)
Tempus’ patient consent form for genomic testing, for international use.
-
PATIENT FORMS
Patient Consent Form (EU/UK)
Tempus’ patient consent form for genomic testing, for international use (EU/UK specific).
-
PATIENT FORMS
Patient Consent Form (Spanish)
Formulario de consentimiento del paciente adjunto al formulario de solicitud principal (solo aplicable en los estados pertinentes).
-
PATIENT FORMS
Tempus nP Digital Patient Brochure (Spanish)
Folleto de información al paciente para los exámenes de Neurología y Psiquiatría de Tempus.
SPECIMEN GUIDELINES
-
SPECIMEN GUIDELINES
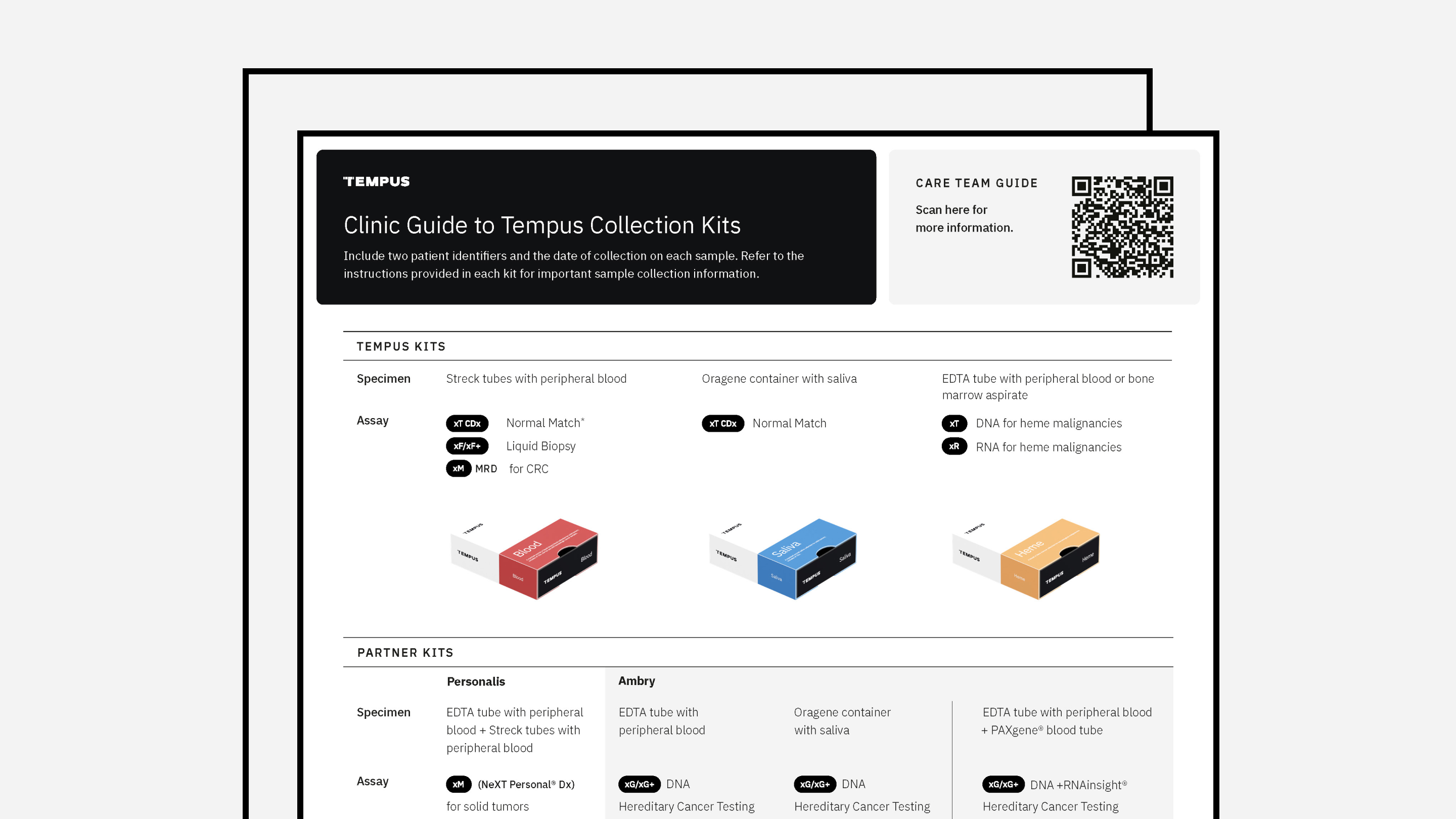
Specimen Guidelines for Oncology
Tempus’ specimen collection guidelines for providers.
-
SPECIMEN GUIDELINES
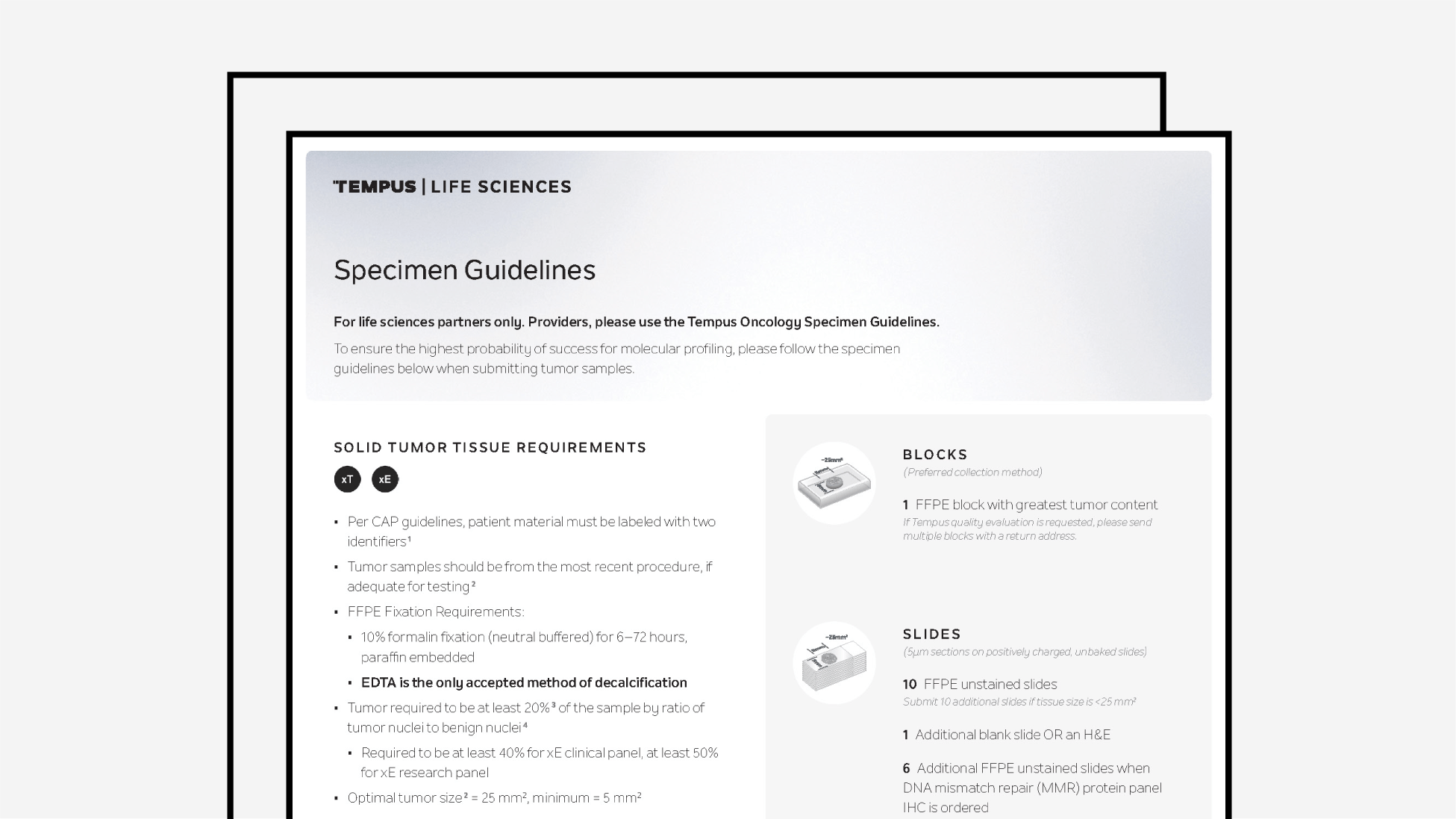
Specimen Guidelines for Life Sciences
Tempus’ specimen collection guidelines for Life Sciences partners only.
-
SPECIMEN GUIDELINES
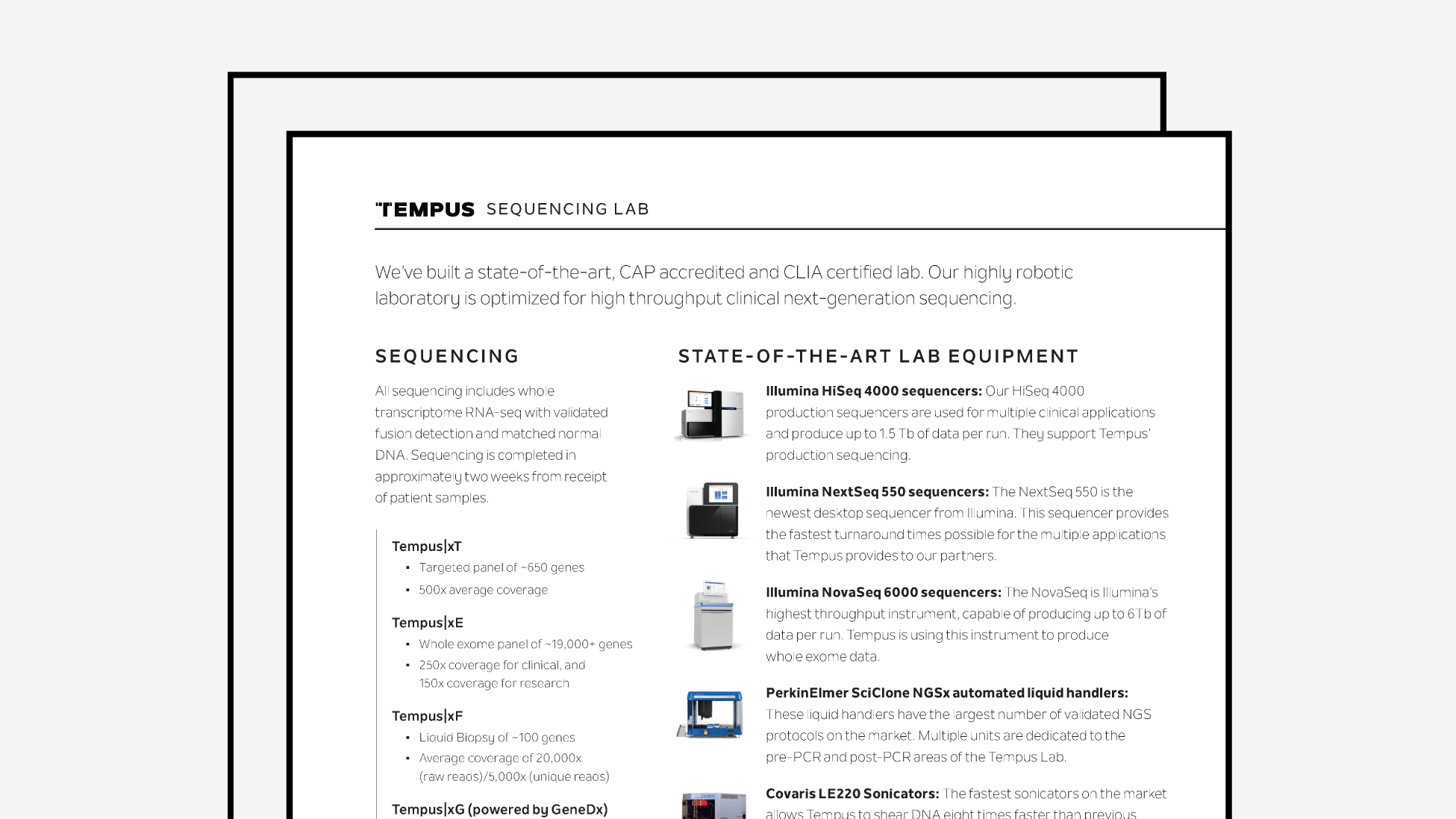
Lab Information Guide
An outline of Tempus’ state-of-the-art CAP-accredited and CLIA-certified sequencing lab equipment.
-
SPECIMEN GUIDELINES
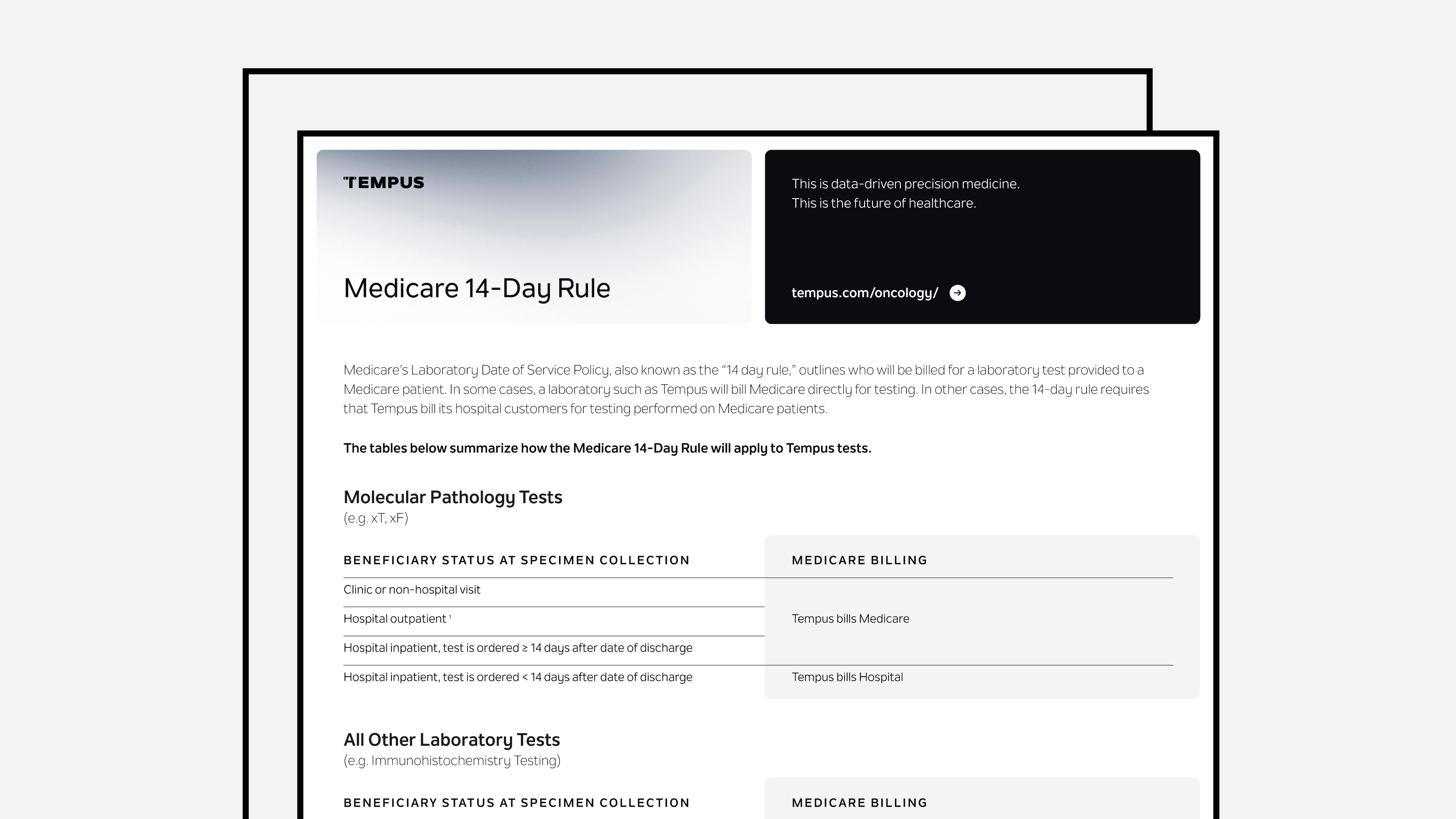
Medicare 14-Day Rule
Medicare's Laboratory Date of Service Policy, also known as the "14 day rule," determines whether Tempus bills Medicare or its hospital customers for laboratory tests provided to Medicare patients.
SAMPLE REPORTS
-
SAMPLE REPORTS
Clinical Report Guide
A guide designed to help you understand Tempus’ clinical reports.
-
SAMPLE REPORTS
Tempus xG Sample Report Guide
A guide designed to help you understand Tempus’ hereditary panels’ clinical reports.
VALIDATIONS & GENE PANELS
-
VALIDATIONS & GENE PANELS
Tempus xT CDx Technical Information
Detailed information regarding the FDA approval of xT CDx.
-
VALIDATIONS & GENE PANELS
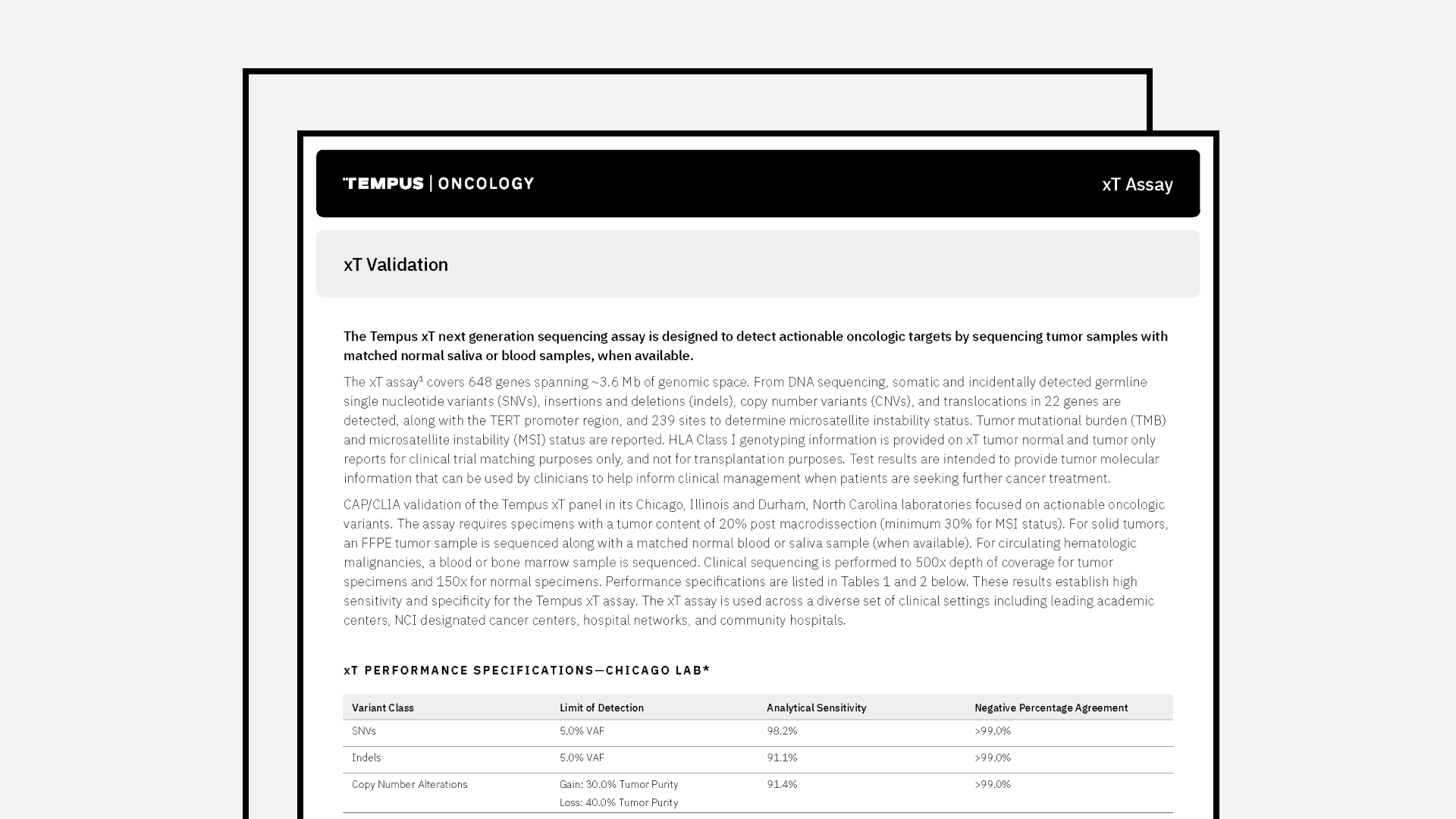
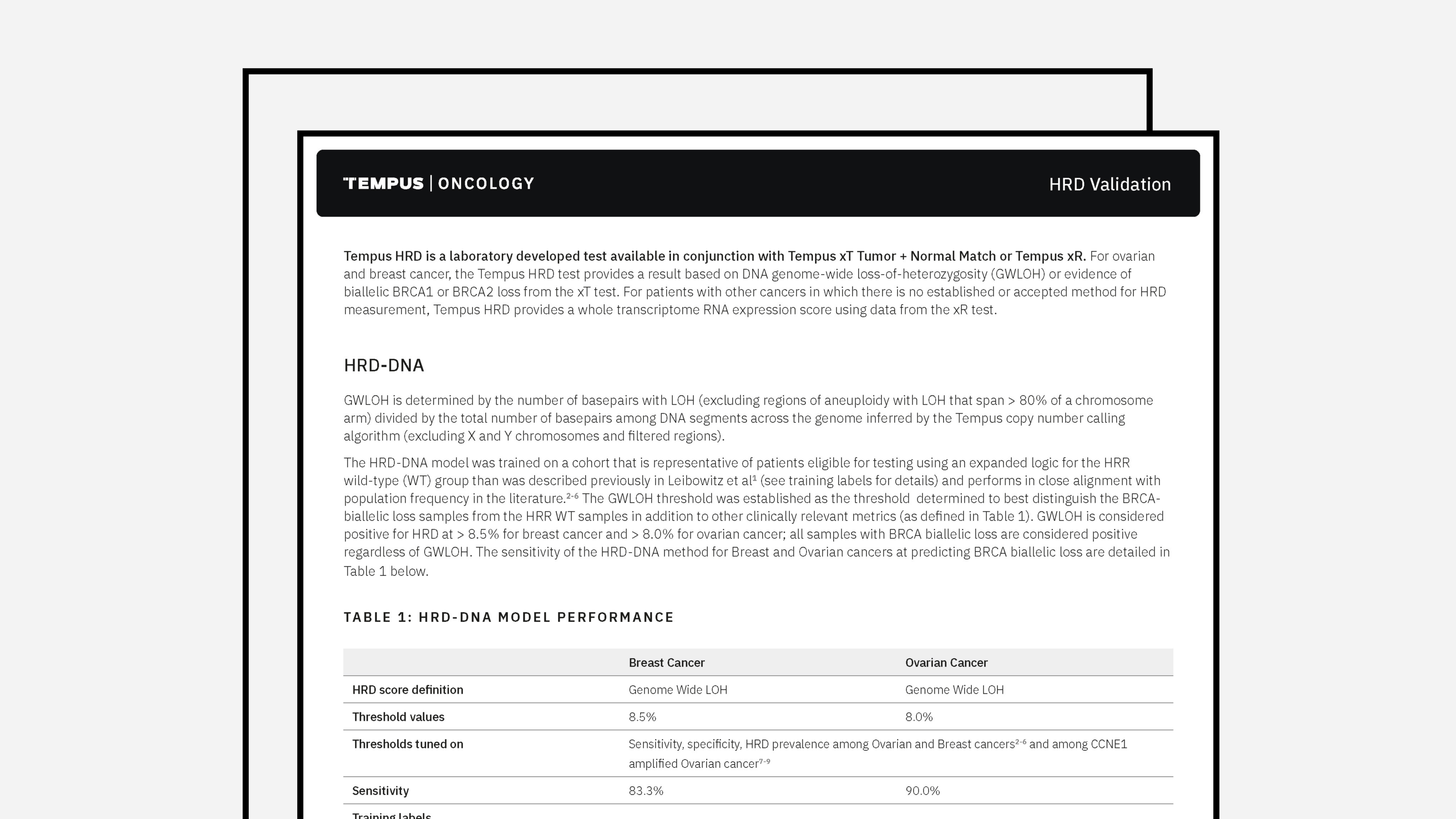
Tempus xT Validation
The combined latest validation summary for Tempus’ xT assay and a panel of genes detected by Tempus’ xT assay.
-
VALIDATIONS & GENE PANELS
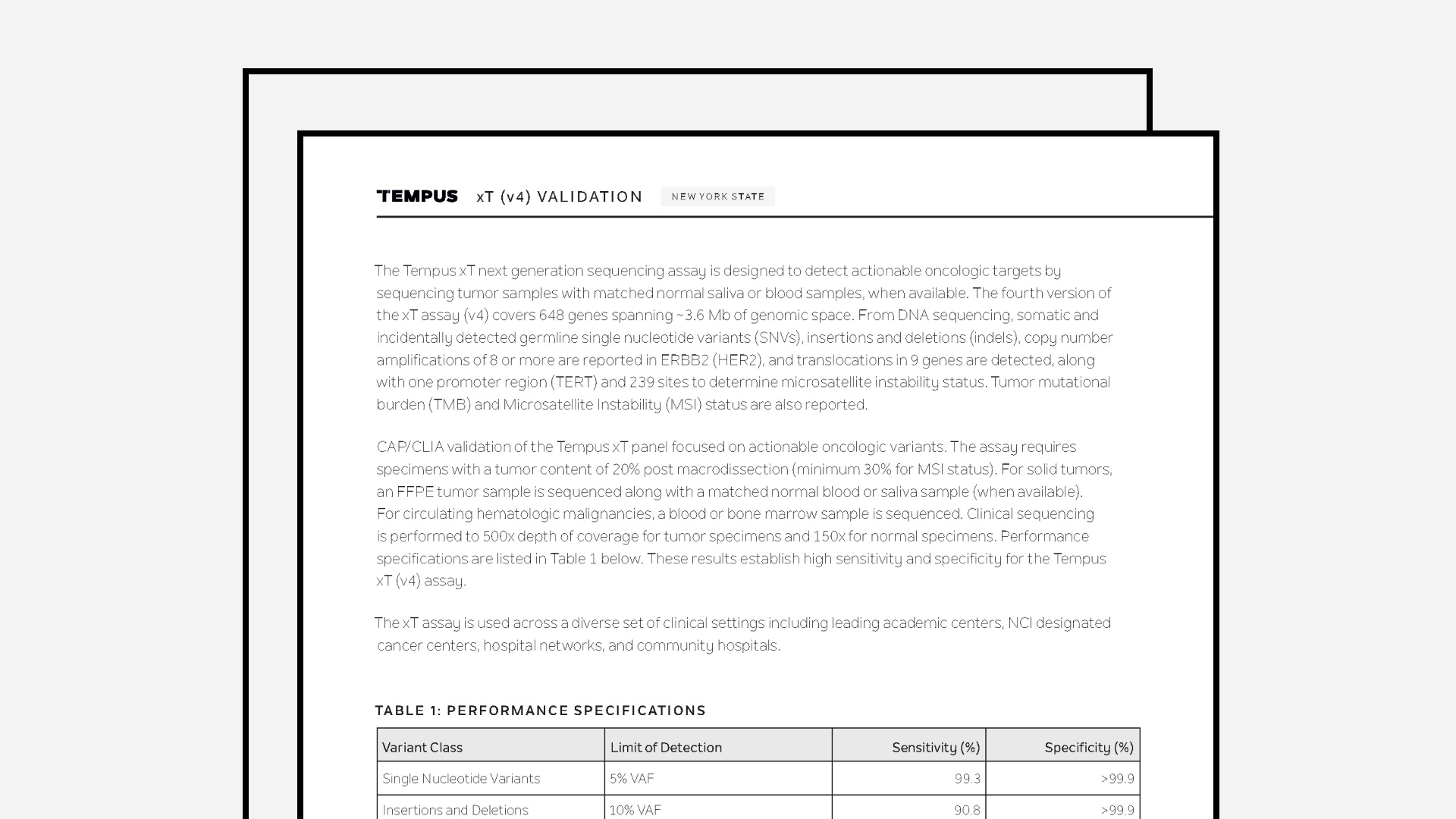
Tempus xT Validation (NYS)
The latest validation summary for Tempus’ xT assay, New York State specific.
-
VALIDATIONS & GENE PANELS
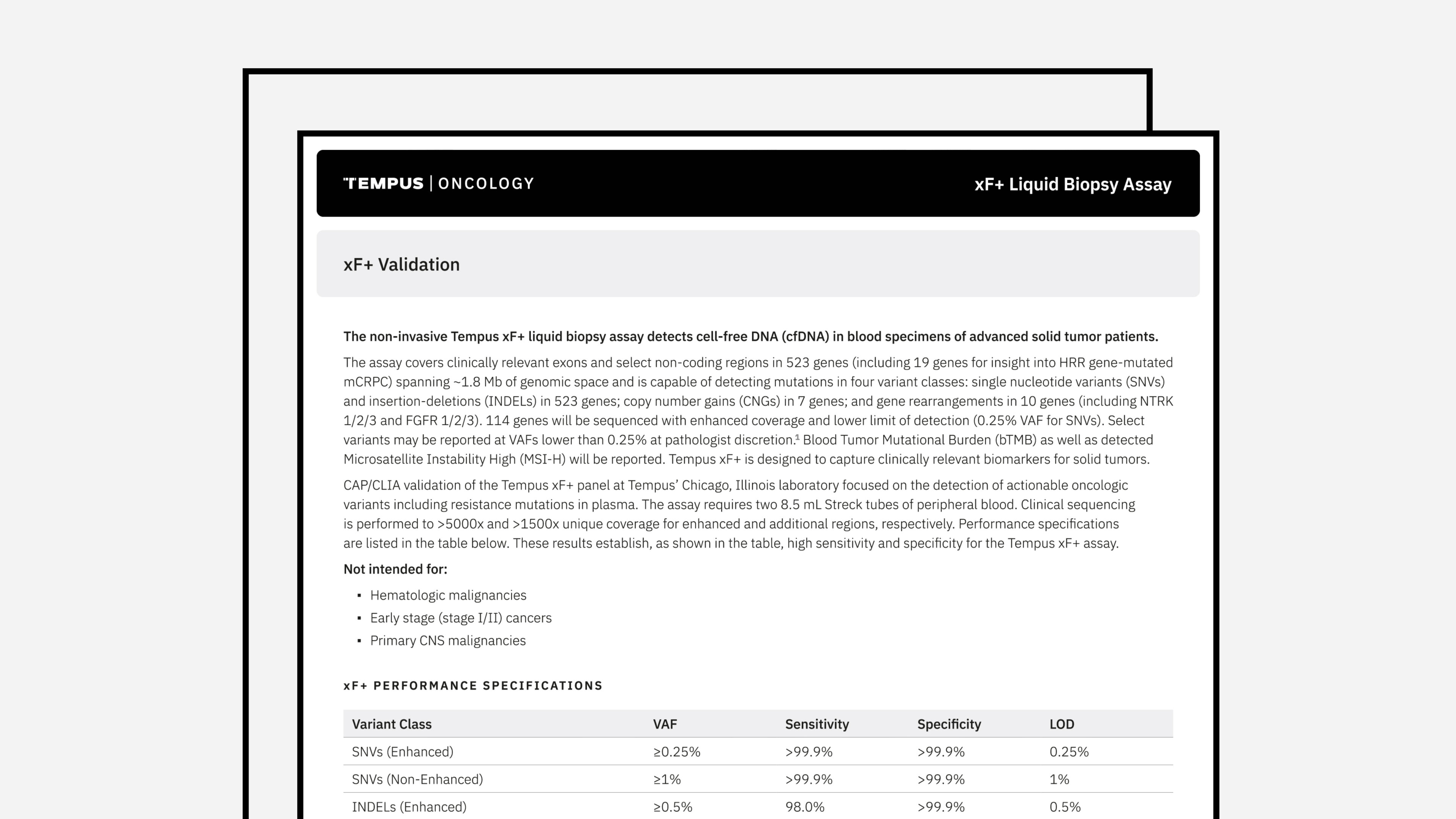
Tempus xF+ Validation
The latest validation summary and panel of genes for Tempus’ xF+ assay.
-
VALIDATIONS & GENE PANELS
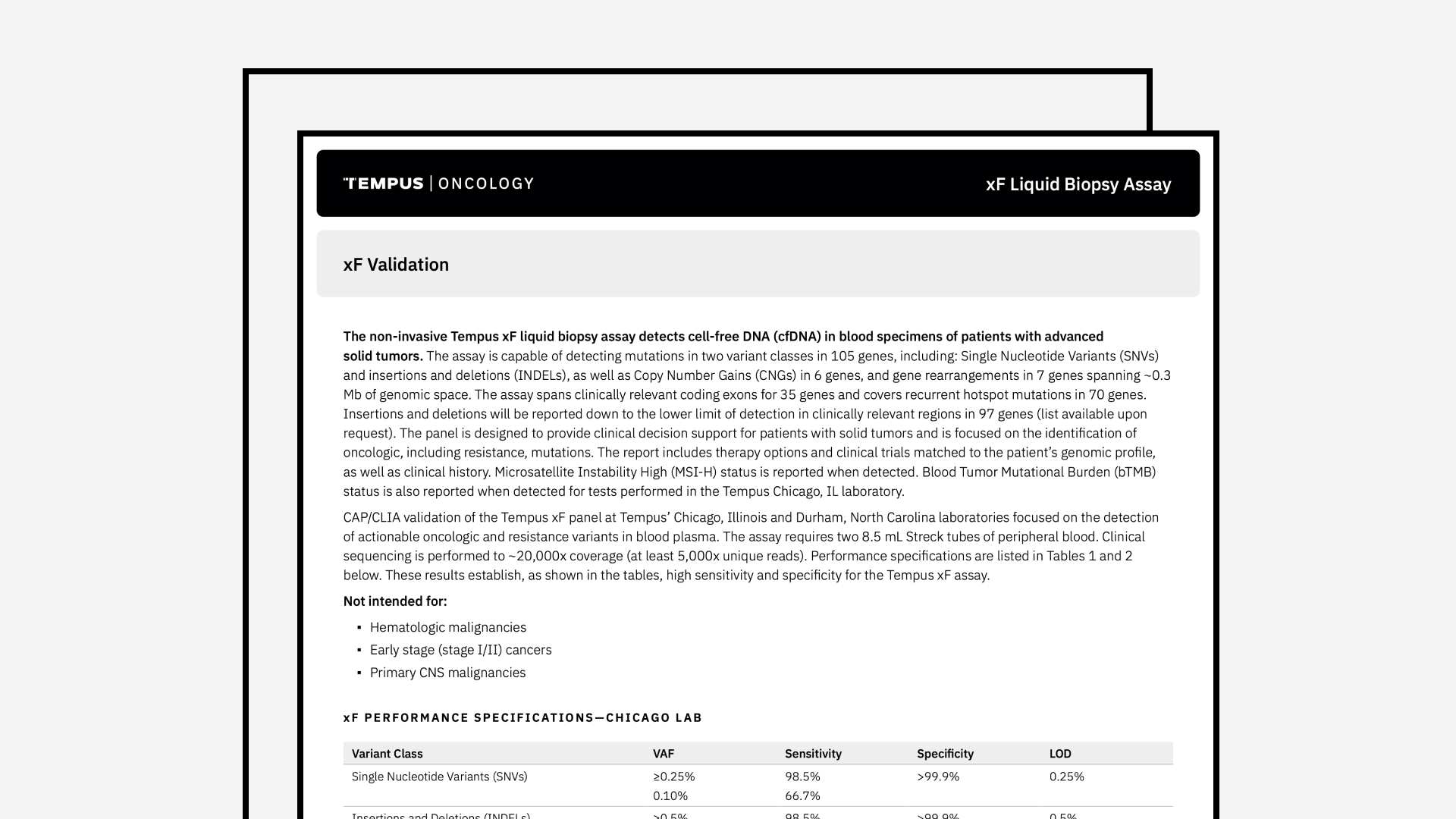
Tempus xF Validation
The latest combined validation summary for Tempus’ xF assay and a panel of genes detected by Tempus’ xF assay.
-
VALIDATIONS & GENE PANELS
Tempus xF Validation (NYS)
The latest validation summary for Tempus’ xF assay, New York State specific.
-
VALIDATIONS & GENE PANELS
Tempus xT Gene Panel (NYS)
A panel of genes detected by Tempus’ xT assay, New York State specific.
-
VALIDATIONS & GENE PANELS
Tempus xF Gene Panel (NYS)
A panel of genes detected by Tempus’ xF assay, New York State specific.
-
VALIDATIONS & GENE PANELS
HLA Genotyping Ambiguous Allele List (v2)
A list of the representative and associated ambiguous alleles reported by Tempus xT’s HLA genotyping feature.
-
VALIDATIONS & GENE PANELS
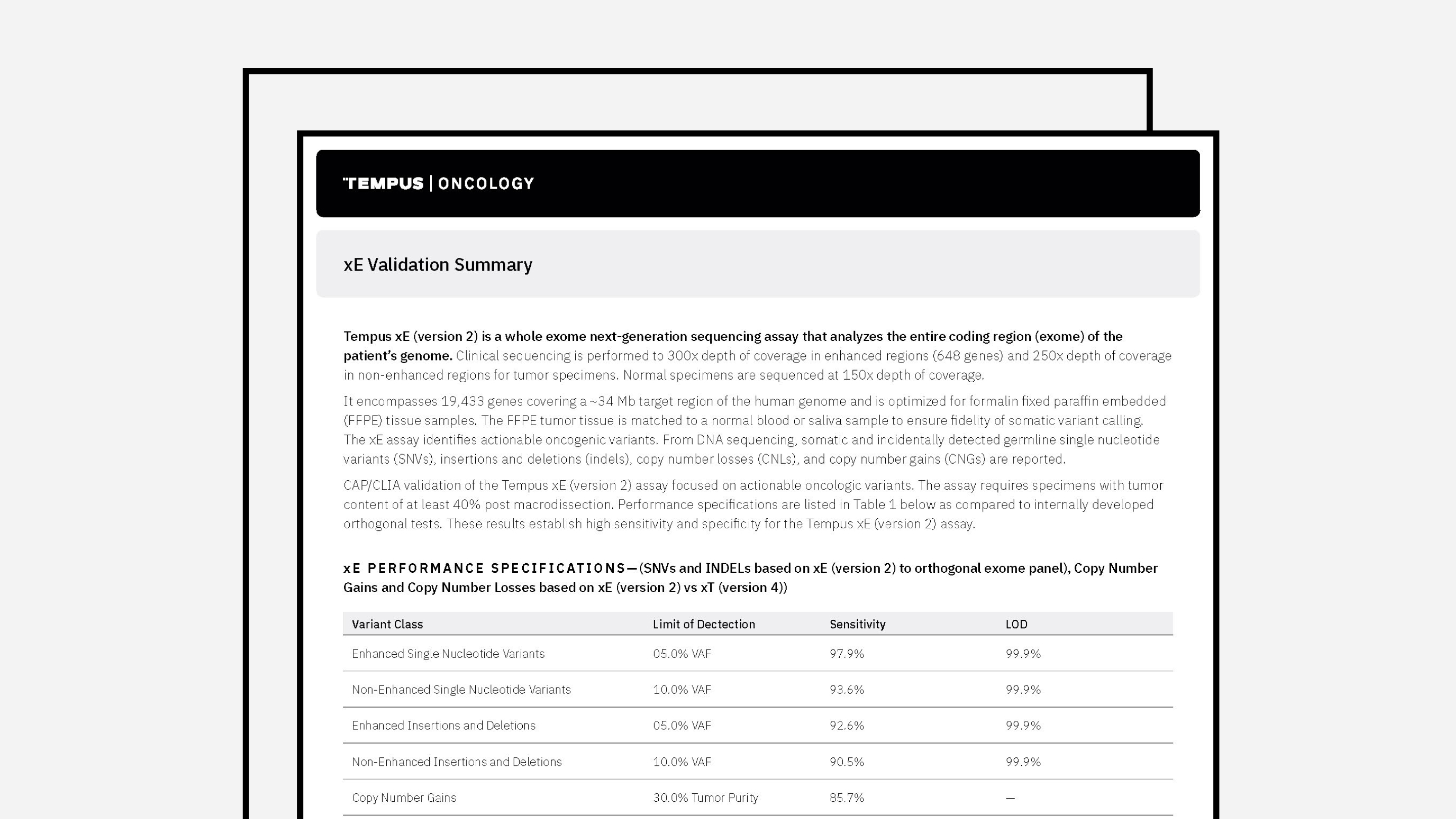
Tempus xE Validation
The latest validation summary and panel of genes for Tempus’ xE assay.
-
VALIDATIONS & GENE PANELS
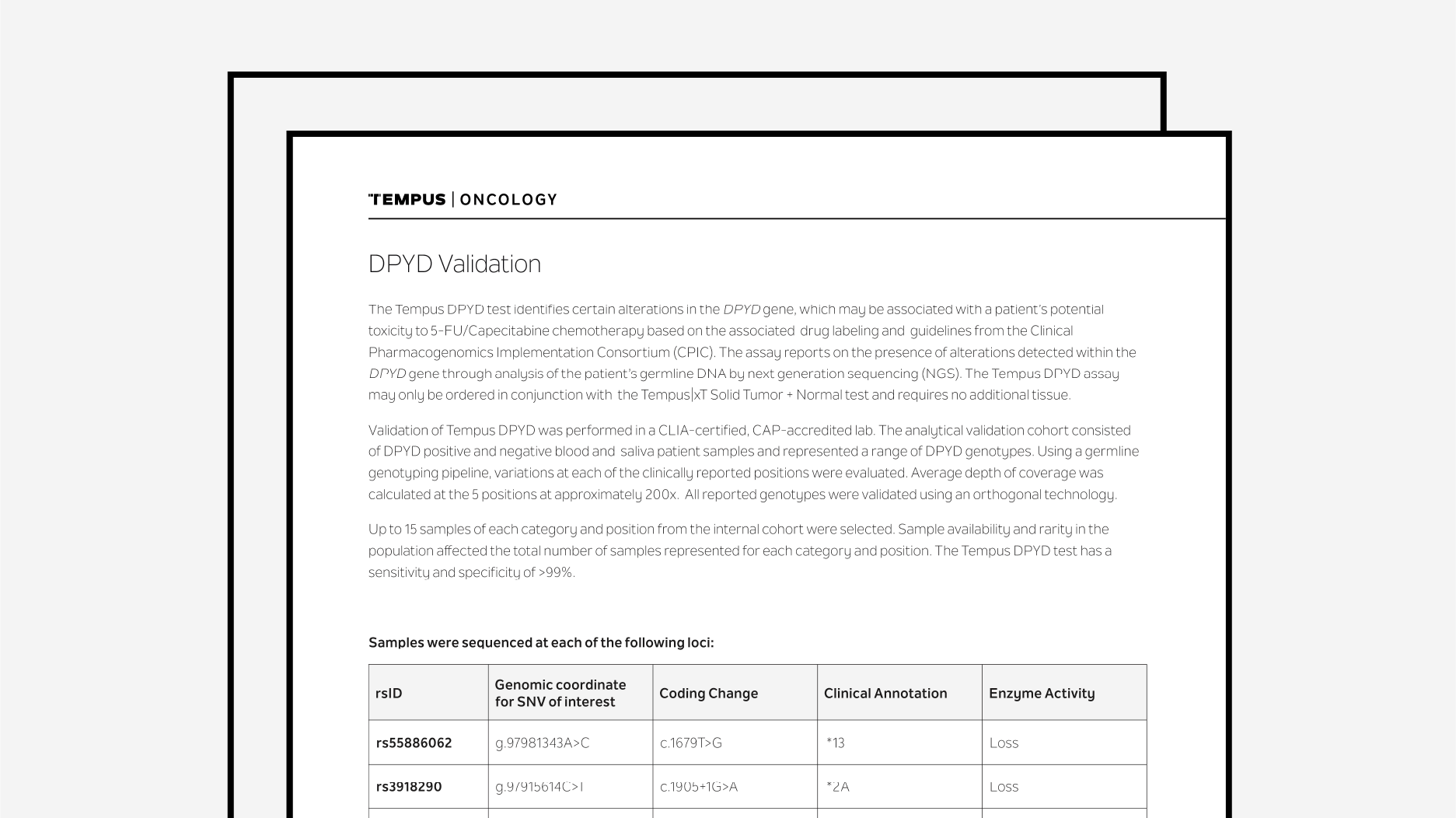
Tempus DPYD Validation
The latest validation summary for Tempus' DPYD test.
-
VALIDATIONS & GENE PANELS
Tempus UGT1A1 Validation
The latest validation summary for Tempus' UGT1A1 test.
-
VALIDATIONS & GENE PANELS
Tempus PurIST Validation
The latest validation summary for Tempus' PurIST test.
-
VALIDATIONS & GENE PANELS
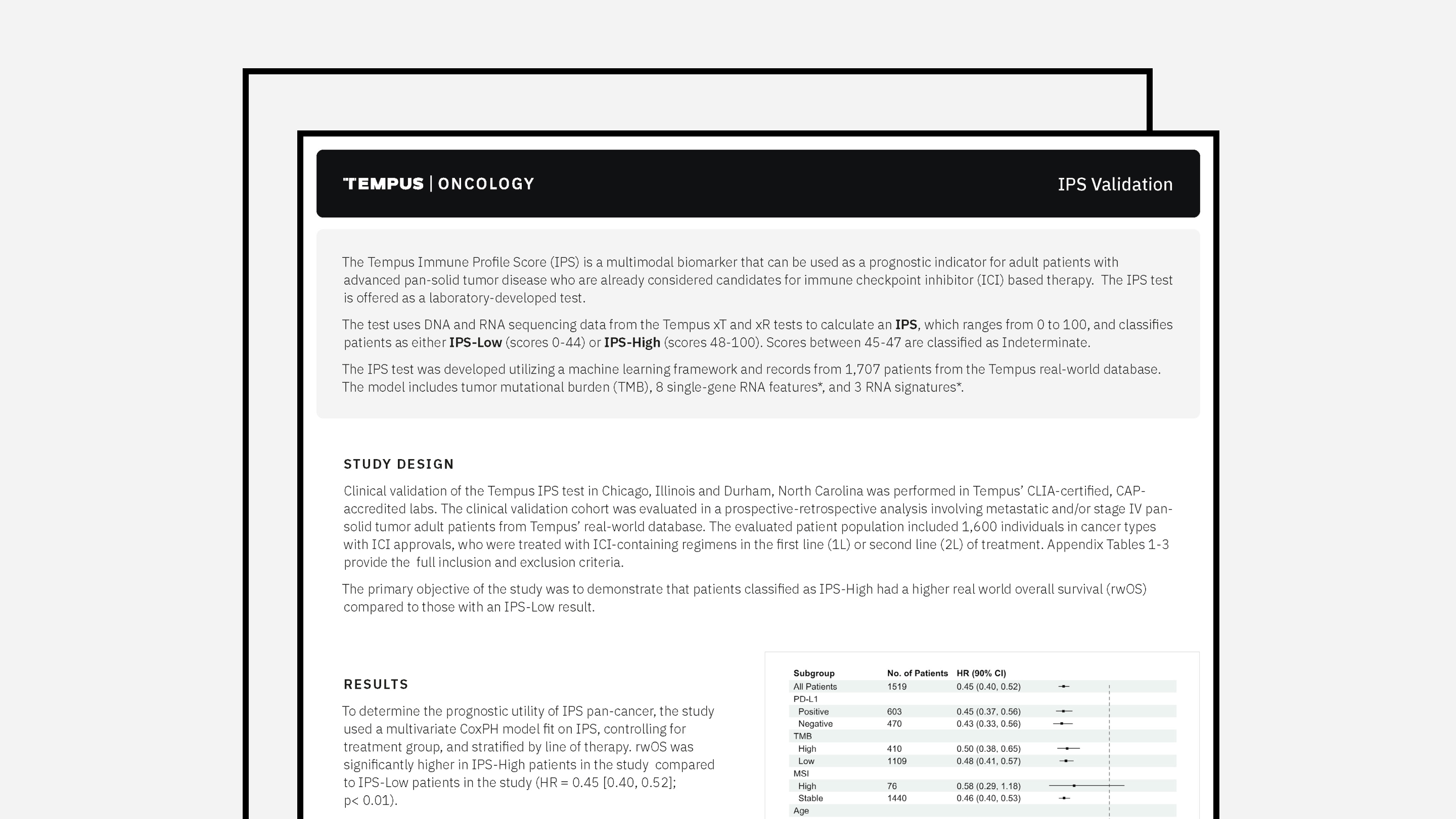
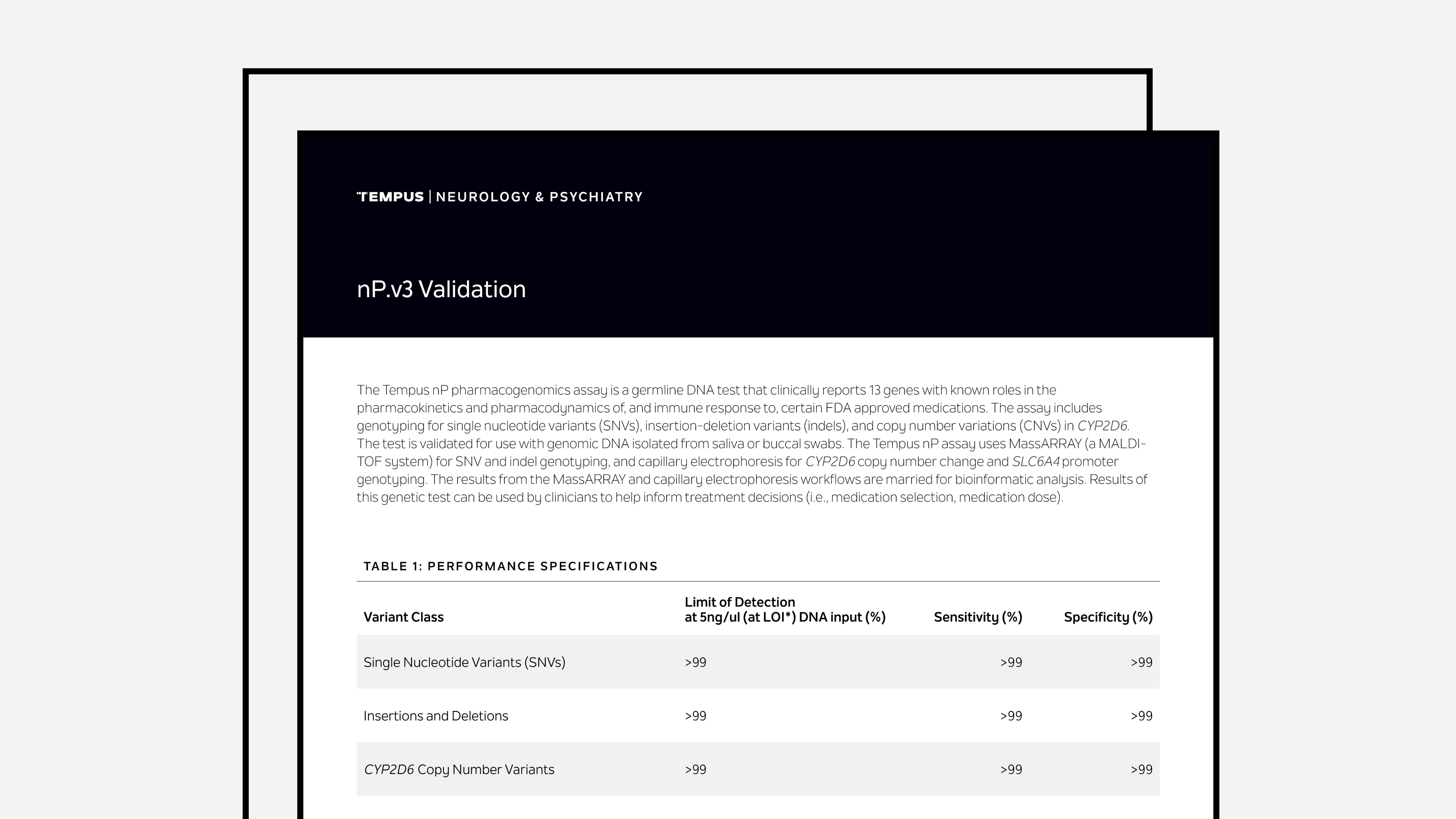
Tempus nP Validation Summary
The latest validation summary for Tempus’ nP PGx assay.