LIFE SCIENCE RESEARCH APPLICATIONS
IPS offers robust support for life sciences research, providing actionable insights in various applications.
IPS is an AI-developed algorithm providing prognostic insights into real-world overall survival (rwOS) outcomes for ICI treatment in metastatic solid tumors.
The information on this page is intended for life sciences partners and focuses on research and development applications. If you would like to learn more about the clinical applications, click here.
Despite advancements in biomarker testing, the need for more sensitive and generalizable biomarkers to predict ICI treatment outcomes still remains. The development of reliable predictors has been hindered by limited access to multi-omic testing and substantial validation cohorts.
IPS helps address these challenges as a DNA- and RNA-based molecular signature that demonstrates prognostic utility in an overall pan-cancer cohort of metastatic solid tumor patients receiving ICI-based regimens.
IPS provides an “Immune Profile Score” ranging from 0 to 100 and classifies patients into two groups*
*Note: Some patients will have Indeterminate results. The Indeterminate range of 45-47 accounts for assay variability; therefore the sample cannot be reliably classified as IPS-High or IPS-Low.
Download the Immune Profile Score (IPS) OverviewA prospective-retrospective validation in 1,519 patients demonstrated strong stratification of overall survival (OS) (hazard ratio [HR]=0.45 [0.41-0.57]) in the overall cohort, showing significantly longer OS in IPS-High patients.
Learn more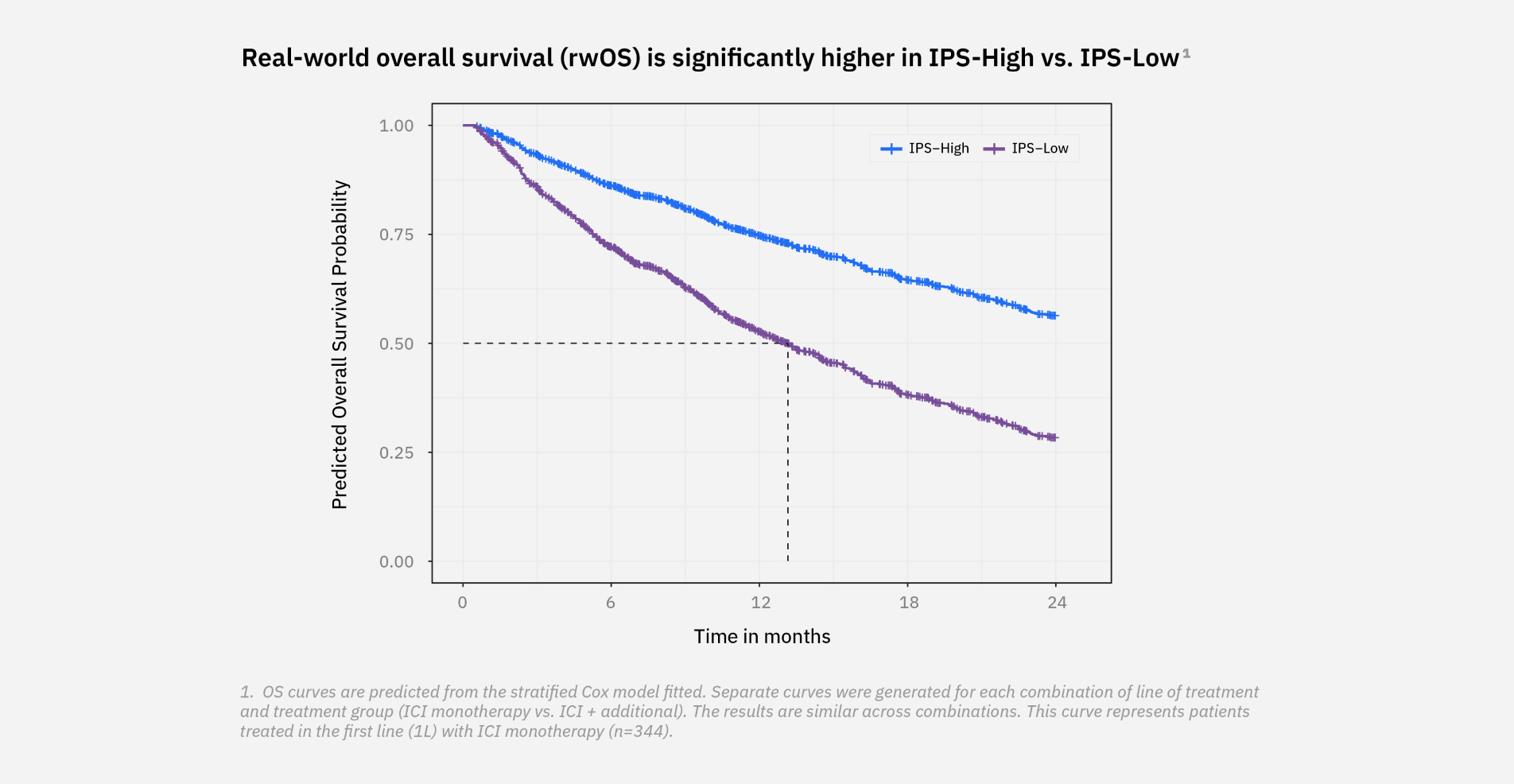
IPS combines DNA and RNA biomarkers for a robust prognostic score.
Applicable to a wide range of solid tumors, supporting broad research applications.
IPS aids in identifying patient populations who may respond favorably to ICI therapies.
IPS can be added to concurrent Tempus xT and xR test orders without requiring additional tissue samples or wet lab assays.
Validated in 1,500+ pan-cancer patients, ensuring the reliability and robustness of the IPS algorithm.
Develop IPS as a companion diagnostic (CDx) for existing or novel therapies and leverage our network of 6,500+ oncologists to help drive clinical adoption.
IPS offers robust support for life sciences research, providing actionable insights in various applications.
We offer a full spectrum of capabilities—from data licensing to validation to adoption—ensuring support for your research needs.
Access IPS results through sequencing of retrospective tissue samples with concurrent Tempus xT and xR orders.
Learn moreAnalyze IPS results across de-identified patient records that incorporate clinical, molecular, and imaging modalities through flexible licensing solutions.
Learn morePartner with us to develop IPS as a CDx for an existing or novel therapy.
Learn moreWith a broad range of laboratory services and data analysis capabilities that support immunotherapy development, Tempus is your comprehensive precision medicine partner.